November 29, 2022 by Chain Drug Review
Aurobindo Pharma
Pharmacy, Supplier News

EAST WINDSOR, N.J. – Aurobindo Pharma Limited has received final approval from U.S. Food and Drug Administration for its Abbreviated New Drug Application carbidopa and levodopa tablets USP, 10mg/100mg, 25mg/100mg, and 25mg/250mg. Aurobindo Pharma’s carbidopa and levodopa tablets, are an AB-rated generic equivalent to the reference listed drug (RLD), SINEMET Tablets, manufactured by ORGANON. Carbidopa
July 27, 2022 by Chain Drug Review
Aurobindo Pharma
Pharmacy, Supplier News

EAST WINDSOR, N.J. – Aurobindo Pharma has received final approval from U.S. Food and Drug Administration for its Abbreviated New Drug Application ambrisentan tablets, 5mg and 10mg. Aurobindo Pharma’s ambrisentan tablets are an AB-rated generic equivalent to the reference listed drug (RLD), LETAIRIS Tablets, manufactured by Gilead Sciences Inc. Ambrisentan tablets are indicated for the
May 23, 2022 by Chain Drug Review
Aurobindo Pharma, exemestane tablets
Pharmacy, Supplier News

EAST WINDSOR, N.J. – Aurobindo Pharma has received final approval from U.S. Food and Drug Administration for its Abbreviated New Drug Application exemestane tablets USP, 25 mg. Aurobindo Pharma’s exemestane tablets are an AB-rated generic equivalent to the reference listed drug (RLD), AROMASIN Tablets, manufactured by Pfizer. Exemestane tablets are indicated for adjuvant treatment of
May 10, 2022 by Chain Drug Review
Aurobindo Pharma
Pharmacy, Supplier News

EAST WINDSOR, N.J. – Aurobindo Pharma has received final approval from U.S. Food and Drug Administration for its Abbreviated New Drug Application vigabatrin tablets USP, 500mg. Aurobindo Pharma’s vigabatrin tablets are an AB-rated generic equivalent to the reference listed drug (RLD), SABRIL tablets, manufactured by Lundbeck Pharmaceuticals LLC. Vigabatrin tablets are indicated as adjunctive therapy
April 1, 2022 by Chain Drug Review
Aurobindo Pharma
Pharmacy, Supplier News

EAST WINDSOR, N.J. – Aurobindo Pharma has received final approval from U.S. Food and Drug Administration for its Abbreviated New Drug Application acyclovir oral suspension USP, 200 mg/5 mL. Aurobindo Pharma’s acyclovir oral suspension are an AB-rated generic equivalent to the reference listed drug (RLD), ZOVIRAX (acyclovir), of Mylan Pharmaceuticals Inc. Acyclovir oral suspension are
March 29, 2022 by Chain Drug Review
Aurobindo Pharma, Bromfenac Ophthalmic Solution
Pharmacy, Supplier News

EAST WINDSOR, N.J. – Aurobindo Pharma has received final approval from U.S. Food and Drug Administration for its Abbreviated New Drug Application Bromfenac Ophthalmic Solution 0.09%. Aurobindo Pharma’s Bromfenac Ophthalmic Solution, are an AT-rated generic equivalent to the reference listed drug (RLD), Bromday Ophthalmic Solution, of Bausch & Lomb. Bromfenac Ophthalmic Solution are indicated for
March 28, 2022 by Chain Drug Review
Aurobindo Pharma, clobazam oral suspension
Pharmacy, Supplier News

EAST WINDSOR, N.J. – Aurobindo Pharma has received final approval from U.S. Food and Drug Administration for its Abbreviated New Drug Application clobazam oral suspension, 2.5m/mL. Aurobindo Pharma’s clobazam oral suspension, are an AB-rated generic equivalent to the reference listed drug (RLD), ONFI Oral Suspension, of Lundbeck Pharmaceuticals. Clobazam oral suspension are indicated for the
February 21, 2022 by Chain Drug Review
Aurobindo Pharma, Digoxin tablets
Pharmacy, Supplier News

EAST WINDSOR, N.J. — Aurobindo Pharma announced that digoxin tablets in 0.62mg strength has been approved by the FDA and is now available. The company also said that this is the first and only generic in the marketplace for this product.
February 14, 2022 by Chain Drug Review
Aurobindo Pharma, Digoxin tablets
Pharmacy, Supplier News

EAST WINDSOR, N.J. – Aurobindo Pharma has received final approval from U.S. Food and Drug Administration for its Abbreviated New Drug Application digoxin tablets USP, 0.0625 mg, 0.125 mg and 0.25 mg. Aurobindo Pharma’s digoxin tablets, are an AB-rated generic equivalent to the reference listed drug (RLD), Lanoxin Tablets USP, of Concordia Pharmaceuticals. Digoxin tablets
January 12, 2022 by Chain Drug Review
Aurobindo Pharma, colchicine tablets USP 0.6 mg.
Pharmacy, Supplier News

EAST WINDSOR, N.J. – Aurobindo Pharma has received final approval from U.S. Food and Drug Administration for its Abbreviated New Drug Application colchicine tablets USP, 0.6 mg. Aurobindo Pharma’s colchicine tablets, are an AB-rated generic equivalent to the reference listed drug (RLD), COLCRYS Tablets, of Takeda Pharmaceuticals USA, Inc. Colchicine tablets are indicated in adults
December 15, 2021 by Chain Drug Review
Aurobindo Pharma
Pharmacy, Supplier News
EAST WINDSOR, N.J. — After many months of hard work and dedication from their marketing and web design team, Aurobindo Pharma announces the launch of their new company website. The new site is said to offer a clean and modern design, easy navigation and helpful tools and resources. Through this launch, Aurobindo strives to deliver
November 18, 2021 by Chain Drug Review
Aurobindo Pharma, prednisone tablets
Pharmacy, Supplier News

EAST WINDSOR, N.J. — Aurobindo Pharma has received final approval from U.S. Food and Drug Administration for its Abbreviated New Drug Application prednisone tablets USP, 1mg. Aurobindo Pharma’s prednisone tablets USP, 1mg, are an AB-rated generic equivalent to the reference listed drug (RLD), Meticorten Tablets, of Schering Corp Sub Schering Plough Corp. Prednisone tablets are
September 30, 2021 by Chain Drug Review
Aurobindo Pharma
Pharmacy, Supplier News

EAST WINDSOR, N.J. – Aurobindo Pharma has received final approval from U.S. Food and Drug Administration for its Abbreviated New Drug Application vigabatrin for oral solution USP, 500mg. Aurobindo Pharma’s vigabatrin for oral solution USP, 500mg, are an AB-rated generic equivalent to the reference listed drug (RLD), Sabril for Oral Solution of Lundbeck Pharmaceuticals. Vigabatrin
September 23, 2021 by Chain Drug Review
Aurobindo Pharma
Pharmacy, Supplier News
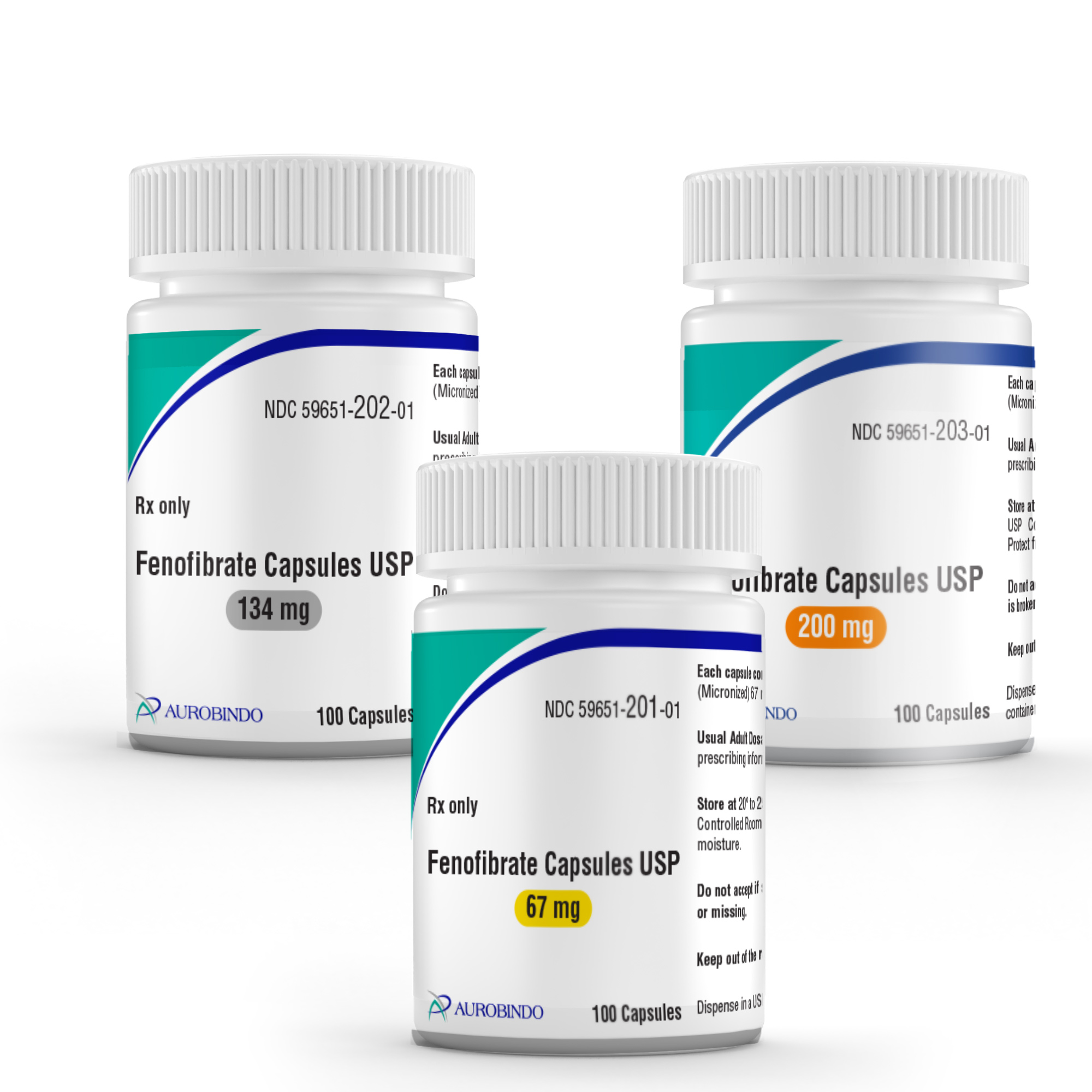
EAST WINDSOR, N.J. – Aurobindo Pharma Limited has received final approval from the U.S. Food and Drug Administration for its Abbreviated New Drug Application fenofibrate capsules USP, 67mg, 134mg, and 200mg. Aurobindo Pharma’s fenofibrate capsules USP, 67mg, 134mg, and 200mg are an AB-rated generic equivalent to the reference listed drug (RLD), Tricor Capsules of AbbVie.





