March 27, 2024 by Chain Drug Review
Dr. Reddy’s, Sanofi
Pharmacy, Supplier News

HYDERABAD, India — Dr. Reddy’s Laboratories Ltd. has entered into an exclusive partnership with Sanofi Healthcare India Private Ltd. to promote and distribute the latter’s vaccine brands across private markets in India. Under the arrangement, Dr. Reddy’s will have exclusive rights to promote and distribute Sanofi’s well established and trusted paediatric and adult vaccine brands
November 17, 2022 by Chain Drug Review
CHPA, Loucks, Sanofi
Leading Headlines, Supplier News

NEW YORK — Andrew Loucks, head of North American consumer healthcare at Sanofi, was elected to the Consumer Healthcare Products Association’s (CHPA) board of directors at its meeting here Thursday. Loucks has been in his current role at Sanofi since his appointment in August. “We very much look forward to Andrew’s participation on the CHPA
June 22, 2022 by Chain Drug Review
Fluzone High- Dose Quadrivalent (Influenza Vaccine), Sanofi
Leading Headlines, Pharmacy
CAMBRIDGE, MA – A majority (66%) of surveyed healthcare providers (HCPs) in the U.S. said that if they could recommend to the CDC one influenza vaccine for adults 65 years and older (65+), they would choose a vaccine with the clinical profile of Fluzone High- Dose Quadrivalent (Influenza Vaccine), according to a survey of 700
November 15, 2021 by Chain Drug Review
Deloitte Consulting, Sanofi
Leading Headlines, Supplier News, Technology

NEW YORK — Deloitte and Sanofi have collaborated on a next-generation, artificial intelligence (AI) software-as-a-service adverse events case intake platform called ConvergeHEALTH Safety to transform pharmacovigilance (PV) and address some of the industry’s biggest operational safety challenges. As an outcome of the initial deployment, Sanofi has already improved case quality and improved case processing efficiencies
January 22, 2021 by Chain Drug Review
Accumulus Synergy, Amgen, and Takeda, Astellas, Bristol-Myers Squibb, GSK, Lilly, Pfizer, Roche, Sanofi, the Janssen Pharmaceutical Companies of Johnson & Johnson (Janssen)
Leading Headlines, Pharmacy

BURLINGAME, Calif. — Ten of the world’s leading biopharmaceutical companies announced the formation of a new non-profit corporation, Accumulus Synergy, which is intended to support interactions between industry and health authorities worldwide to enable real-time collaboration and data exchange, as well as data submission. Accumulus Synergy was formed on July 13, 2020, to develop a
October 18, 2019 by Chain Drug Review
ranitidine, Sanofi, Zantac
Leading Headlines, Pharmacy

BRIDGEWATER, N.J.— Sanofi announced on Friday that it was recalling the over-the-counter heartburn drug Zantac in the United States and Canada, a month after the Food and Drug Administration first alerted the public that versions of the drug contained low levels of a cancer-causing contaminant. Several manufacturers of generic versions of Zantac, ranitidine, had already recalled their
October 22, 2018 by Chain Drug Review
Dupixent (dupilumab), eosinophilic phenotype or with oral corticosteroid-dependent asthma, George Yancopoulos, Olivier Brandicourt, Regeneron, Sanofi
Pharmacy, Supplier News

TARRYTOWN, N.Y. and PARIS — Regeneron Pharmaceuticals, Inc. and Sanofi today announced the U.S. Food and Drug Administration has approved Dupixent (dupilumab) as an add-on maintenance therapy in patients with moderate-to-severe asthma aged 12 years and older with an eosinophilic phenotype or with oral corticosteroid-dependent asthma. Dupixent inhibits the overactive signaling of interleukin-4 (IL-4) and interleukin-13 (IL-13),
April 5, 2018 by Chain Drug Review
diabetes, Insulins VALyou Savings Program, Michelle Carnahan, Sanofi
Pharmacy, Supplier News

BRIDGEWATER, N.J. — Sanofi’s new Insulins VALyou Savings Program will seek to help lower out-of-pocket costs for people living with diabetes who otherwise would pay full retail price for Lantus or Admelog. This includes certain uninsured patients who don’t qualify for traditional patient assistance programs, in addition to some commercially insured patients with a high
January 22, 2018 by Chain Drug Review
Alprolix, Bioverativ, Eloctate, hemophilia treatment market, John Cox, Olivier Brandicourt, Sanofi
Business, Pharmacy, Supplier News

PARIS — Sanofi plans to buy biopharmaceutical company Bioverativ Inc. in a deal valued at $11.6 billion. Waltham, Mass.-based Bioverativ specializes in therapies for hemophilia and other rare blood disorders. Sanofi said Monday that the addition of Bioverativ will boost its presence in specialty care and leadership in rare diseases as well as provide an expansion platform
December 11, 2017 by Chain Drug Review
Admelog, insulin lispro, Sanofi
Pharmacy, Supplier News

PARIS — Sanofi has received Food and Drug Administration approval for Admelog, described as the first follow-on insulin lispro to help people with diabetes manage blood sugar levels at mealtime. Sanofi said Monday that Admelog (insulin lispro injection, 100 units/ml) will be available in both vials and the SoloStar pen, which is the most-used disposable
April 3, 2017 by Chain Drug Review
ACT Kids, ACT Kids Toothpaste, Chattem, Jennifer Cooper, Sanofi, Sanofi Consumer Health Care
Supplier News

BRIDGEWATER, N.J. — Sanofi has launched ACT Kids Toothpaste, complementing the ACT brand’s lineup of oral rinses for children. Sanofi said Monday that ACT Kids Toothpaste is an anti-cavity fluoride toothpaste specially designed for children ages 2 and older. The toothpaste strengthens and protects developing teeth and helps prevent cavities. With ACT Kids Toothpaste’s Bubble
February 1, 2017 by Chain Drug Review
levocetirizine, Robert Long, Sanofi, Xyzal Allergy 24HR
Pharmacy, Supplier News

BRIDGEWATER, N.J. — Sanofi is adding to its roster of prescription-to-OTC allergy medications with Xyzal Allergy 24HR. The Food and Drug Administration has approved Xyzal Allergy 24HR (levocetirizine dihydrochloride) as an over-the-counter treatment to alleviate symptoms of seasonal and year-round allergies, Sanofi said Wednesday. The product offers proven 24-hour relief from runny nose, sneezing, itchy
January 4, 2017 by Chain Drug Review
insulin injection, Peter Guenter, Sanofi, Soliqua 100/33, type 2 diabetes
Pharmacy, Supplier News
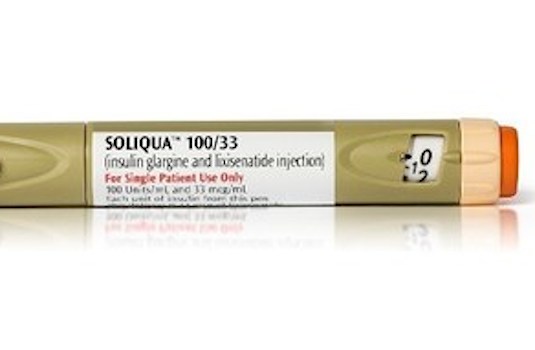
PARIS — Sanofi has released Soliqua 100/33, an insulin injection for type 2 diabetes, to U.S. pharmacies. The company said Wednesday that Soliqua 100/33 (insulin glargine 100 units/ml and lixisenatide 33 mcg/ml) comes in a single, prefilled SoloStar pen with a dosage range of 15 to 60 units and two starting doses to support patients’
October 3, 2016 by Chain Drug Review
Alan Main, Bayer, Boehringer Ingelheim, consumer health care, Olivier Brandicourt, Sanofi
Business, Pharmacy, Supplier News

PARIS — Sanofi has appointed former Bayer executive Alan Main as executive vice president of its newly formed Consumer HealthCare global business unit. Sanofi said Friday that Main, who also joins its executive committee, will be responsible for building and maintaining the company’s position in the consumer health care market, including the expected integration of














