March 4, 2019 by Chain Drug Review
authorized generic of Flector 1 Patch, Brendan O’Grady, Diclofenac epolamine topical patch, Teva Pharmaceutical Industries
Pharmacy, Supplier News

JERUSALEM — Teva Pharmaceutical Industries announced the launch of an authorized generic of Flector 1 Patch, 1.3%, in the U.S. Diclofenac epolamine topical patch, 1.3%, a non-steroidal anti-inflammatory drug (NSAID), is indicated for the topical treatment of acute pain due to minor strains, sprains and contusions. “The launch of our authorized generic of Flector Patch
November 27, 2018 by Chain Drug Review
Brendan O’Grady, piPen Jr (epinephrine injection, Teva Pharmaceutical Industries, USP
Pharmacy, Supplier News

PARSIPPANY, N.J. — Teva Pharmaceutical Industries announced the release of limited doses of the FDA-approved generic version of EpiPen (epinephrine injection, USP) Auto-Injector, 0.3 mg, in the U.S. Teva’s generic version of the EpiPen Jr (epinephrine injection, USP) Auto-Injector, 0.15 mg, and an additional supply of Teva’s generic version of the EpiPen (epinephrine injection, USP) Auto-Injector,
February 20, 2018 by Chain Drug Review
generic versions of Solodyn, Impax Laboratories, minocycline HCl extended-release tablets, Teva Pharmaceutical Industries
Pharmacy, Supplier News
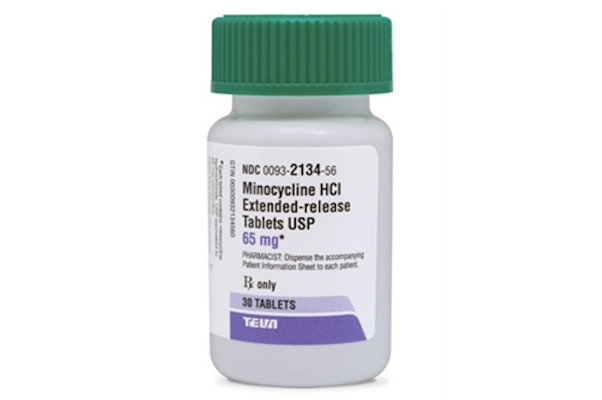
NEW YORK — Teva Pharmaceutical Industries Ltd. and Impax Laboratories Inc. have released minocycline HCl extended-release tablets, an acne medication, in 65 mg and 115 mg dosages. The minocycline products are generic versions of Solodyn (minocycline HCl ER tablets, 65 mg and 115 mg) from Ortho Dermatologics, a unit of Valeant Pharmaceuticals International Inc. Impax’s minocycline product is an authorized generic. An oral antibiotic, minocycline HCl
February 14, 2018 by Chain Drug Review
beclomethasone dipropionate, Brendan O’Grady, QVAR, QVAR RediHaler, Teva Pharmaceutical Industries
Pharmacy, Supplier News
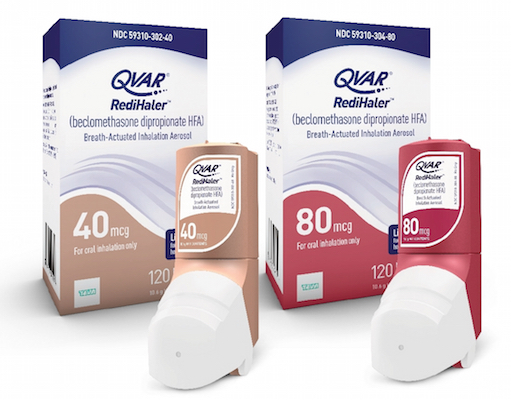
JERUSALEM, Israel — Teva Pharmaceutical Industries Ltd. has made its QVAR RediHaler Inhalation Aerosol (beclomethasone dipropionate HFA) commercially available in 40-mcg and 80-mcg strengths in the United States. The QVAR RediHaler is the first and only breath-actuated aerosol inhaled corticosteroid for the maintenance treatment of asthma as a prophylactic therapy in patients ages 4 years and older,
December 12, 2017 by Chain Drug Review
Brendan O’Grady, generic versions of Viagra, generic Viagra, Greenstone, mylan, Pfizer, sildenafil citrate tablets, Teva Pharmaceutical Industries, Viagra generic
Leading Headlines, Pharmacy, Supplier News

NEW YORK — The first generic versions of Viagra, Pfizer Inc.’s blockbuster drug for erectile dysfunction, were launched this week in the United States by Teva Pharmaceutical Industries Ltd. and Greenstone LLC. Israel-based Teva said it’s offering sildenafil citrate tablets in dosages of 25 mg, 50 mg and 100 mg. Greenstone, a Peapack, N.J.-based Pfizer subsidiary,
November 27, 2017 by Chain Drug Review
Brendan O’Grady, Dipankar Bhattacharjee, Gianfranco Nazzi, Hafrun Fridriksdottir, Kåre Schultz, Michael Hayden, Michael McClellan, Richard Daniell, Rob Koremans, Sven Dethlefs, Teva Pharmaceutical Industries, Teva reorganization
Business, Featured Articles, Leading Headlines, Pharmacy, Supplier News

JERUSALEM, Israel — Just over two months after naming a new chief executive officer, Teva Pharmaceutical Industries Ltd., the world’s largest maker of generic drugs, is reorganizing its business and leadership structure. Teva said Monday that it’s integrating its drug businesses into one commercial organization, and there will no longer be two separate global
November 21, 2017 by Chain Drug Review
Foundation Consumer Healthcare, Greg Bradley, Plan B One-Step, Teva Pharmaceutical Industries
Business, Supplier News

PITTSBURGH — Foundation Consumer Healthcare (FCH) has named Greg Bradley president and chief executive officer. The move comes as Foundation Consumer Healthcare has completed its $675 million acquisition of Plan B One-Step and two value emergency contraceptive brands from Teva Pharmaceutical Industries Ltd. “As the No. 1 OB/GYN-recommended O-T-C emergency contraceptive brand and an important birth
September 11, 2017 by Chain Drug Review
Erez Vigodman, Kåre Schultz, Sol Barer, Teva Pharmaceutical Industries, Yitzhak Peterburg
Business, Leading Headlines, Pharmacy, Supplier News

JERUSALEM, Israel — Kåre Schultz is joining Teva Pharmaceutical Industries Ltd. as president and chief executive officer. Teva said Monday that Schultz will take the reins from interim CEO Yitzhak Peterburg, who took on that role when Erez Vigodman stepped down as president and chief executive earlier this year. Peterburg had been Teva’s chairman but
June 14, 2017 by Chain Drug Review
Dipankar Bhattacharjee, ezetimibe tablets, generic version of Zetia, Teva Global Generic Medicines, Teva Pharmaceutical Industries
Pharmacy, Supplier News
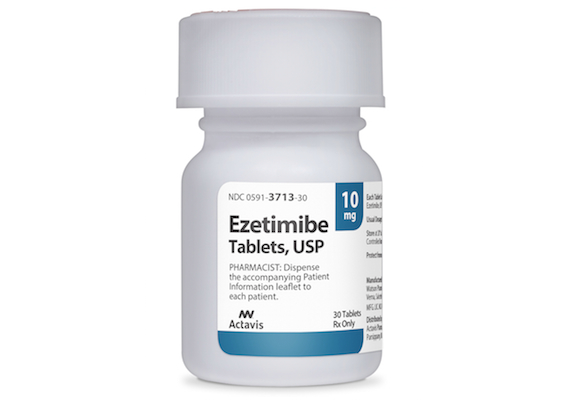
JERUSALEM, Israel — Teva Pharmaceutical Industries Ltd. has released ezetimibe tablets 10 mg, a cholesterol-lowering medication, in the United States. Teva said its ezetimibe product is a generic version of Zetia tablets from Merck & Co. Ezetimibe tablets work to reduce the amount of cholesterol the body absorbs. The drug is used to lower levels
May 15, 2017 by Chain Drug Review
generic version of Glumetza, medication for type 2 diabetes, metformin hydrochloride extended-release tablets, Teva Pharmaceutical Industries, Valeant Pharmaceuticals
Pharmacy, Supplier News

JERUSALEM, Israel — Teva Pharmaceutical Industries Ltd. has launched metformin hydrochloride extended-release tablets, a medication for type 2 diabetes, in the United States. Teva said Monday that its metformin product, available in 500-mg and 1,000-mg dosages, is a generic version of Glumetza tablets from Valeant Pharmaceuticals International. Metformin hydrochlorideER tablets are indicated for use with
March 21, 2017 by Chain Drug Review
generic version of Minastrin 24 Fe, norethindrone acetate and ethinyl estradiol tablets, oral contraceptive, Teva Pharmaceutical Industries
Pharmacy, Supplier News

JERUSALEM, Israel — Teva Pharmaceutical Industries Ltd. has launched norethindrone acetate and ethinyl estradiol tablets and ferrous fumarate tablets 1 mg/20 mcg, a birth control pill, in the United States. Teva said its product is an authorized generic version of Minastrin 24 Fe tablets from Allergan Pharmaceuticals International Ltd. A combination medicine, Minastrin 24 Fe
February 7, 2017 by Chain Drug Review
Erez Vigodman, Sol Barer, Teva Pharmaceutical Industries, Yitzhak Peterburg
Business, Leading Headlines, Pharmacy, Supplier News

JERUSALEM, Israel — Erez Vigodman is stepping down as president and chief executive officer of Teva Pharmaceutical Industries Ltd. Teva said late Monday that it has appointed chairman Yitzhak Peterburg as interim president and CEO, effective immediately. In addition, Teva’s board of directors has elected Sol Barer, a board member since January 2015, as chairman.
January 20, 2017 by Chain Drug Review
abuse deterrence technology, hydrocodone, Michael Hayden, opioid pain medication, prescription drug abuse, Rob Koremans, Teva Pharmaceutical Industries, Vantrela ER
Pharmacy, Supplier News

JERUSALEM, Israel — Teva Pharmaceutical Industries Ltd. has received Food and Drug Administration approval for Vantrela ER, an opioid pain medication with Teva’s abuse deterrence technology. Vantrela ER (hydrocodone bitartrate extended-release tablets, CII) is indicated for the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment and for which other treatment options
December 6, 2016 by Chain Drug Review
Dipankar Bhattacharjee, Erez Vigodman, Sigurdur Olafsson, Teva Generics Europe, Teva Global Generic Medicines Group, Teva Pharmaceutical Industries
Business, Pharmacy, Supplier News

JERUSALEM, Israel — Dipankar Bhattacharjee has been promoted to president and chief executive officer of the Global Generic Medicines Group at Teva Pharmaceutical Industries Ltd. The appointment is effective immediately. Bhattacharjee, who has served as president and CEO of Teva Generics Europe since April 2013, succeeds Sigurdur Olafsson, who will step down from his role












