November 3, 2022 by Chain Drug Review
Amneal Pharmaceuticals, leuprolide acetate for injection
Pharmacy, Supplier News

BRIDGEWATER, N.J. – Amneal Pharmaceuticals announced that it has received Abbreviated New Drug Application (ANDA) approval from the U.S. Food and Drug Administration for leuprolide acetate for injection. Harsher Singh, SVP for Amneal Biosciences stated, “We are making tremendous progress expanding our injectables business. This latest new product is another key therapeutic for the institutional
October 5, 2022 by Chain Drug Review
Alymsys, Amneal Pharmaceuticals
Pharmacy, Supplier News

BRIDGEWATER, N.J. — Amneal Pharmaceuticals announced the commercial launch of Alymsys (bevacizumab-maly), a biosimilar referencing Avastin. Alymsys is a vascular endothelial growth factor inhibitor used in oncology. This product was developed by mAbxience, a global biotech company with over a decade of experience in the development, manufacture, and commercialization of biopharmaceuticals. “The launch of Alymsys
October 27, 2021 by Chain Drug Review
Amneal Pharmaceuticals, Decadron, dexamethasone
Pharmacy, Supplier News
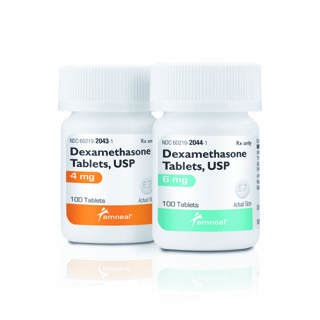
BRIDGEWATER, N.J. — Amneal Pharmaceuticals announced on Wednesday that it is launching dexamethasone, 4mg and 6 mg, following Abbreviated New Drug Application (ANDA) approval from the U.S. Food and Drug Administration. Dexamethasone, the generic version of Decadron, is a well-established anti-inflammatory glucocorticoid steroid used to treat several medical conditions, including respiratory complications associated with COVID-19.
August 5, 2021 by Chain Drug Review
Amneal Pharmaceuticals, taysofy
Pharmacy, Supplier News

BRIDGEWATER, N.J. — Amneal Pharmaceuticals has launched norethindrone acetate and ethinyl estradiol capsules and ferrous fumarate capsules 1 mg/20 mcg following the July 20th approval of its Abbreviated New Drug Application (ANDA) from the U.S. Food and Drug Administration. The Amneal product, marketed under the proprietary of taysofy, is an AB-rated generic equivalent for the
November 11, 2020 by Chain Drug Review
5%, acyclovir cream, Amneal Pharmaceuticals
Pharmacy, Supplier News

BRIDGEWATER, N.J. —Amneal Pharmaceuticals announced that it has received approval for its Abbreviated New Drug Application (ANDA) from the U.S. Food and Drug Administration (FDA) for acyclovir cream, 5%. Acyclovir cream, 5%, is the generic version of Zovirax for treatment of recurrent herpes labialis (cold sores) in immunocompetent adults and adolescents 12 years of age
September 9, 2020 by Chain Drug Review
Amneal Pharmaceuticals, Joe Todisco, nizatidine oral solution
Pharmacy, Supplier News

BRIDGEWATER, N.J. — Amneal Pharmaceuticals announced that its nizatidine oral solution 15 mg/mL (75 mg/5 mL) is now available for reorder. The drug, the generic equivalent of Axid, is the only oral liquid form of nizatidine available in the US. The Amneal product meets all FDA requirements and specification limits related to NDMA. “Nizatidine has
April 17, 2020 by Chain Drug Review
Amneal Pharmaceuticals, Butrans (buprenorphine) Transdermal System, Chirag Patel and Chintu Pate
Pharmacy, Supplier News

BRIDGEWATER, N.J. — Amneal Pharmaceuticals announced that it has received Abbreviated New Drug Application (ANDA) approval from the U.S. Food and Drug Administration (FDA) for a generic version of Butrans (buprenorphine) Transdermal System, 5 mcg/hr, 7.5 mcg/hr, 10 mcg/hr, 15 mcg/hr and 20 mcg/hr. In addition, Amneal was granted the Competitive Generic Therapy (CGT) designation
July 22, 2019 by Chain Drug Review
Alembic, Alkem, Amneal Pharmaceuticals, Dr. Reddy’s, generic version of Lyrica, InvaGen, MSN Laboratories, pregabalin capsules, Rising Pharmaceuticals, Rob Stewart, Sciegen, Teva
Pharmacy, Supplier News
BRIDGEWATER, N.J. — The Food and Drug Administration has approved a number of applications for the generic version of Pfizer’s Lyrica (pregabalin). The FDA gave the green light to the following companies: Amneal, Alembic, Alkem, Dr. Reddy’s, InvaGen, MSN Laboratories, Rising Pharmaceuticals, Sciegen, and Teva. Amneal’s generic version of Lyrica (pregabalin capsules) will be available
July 11, 2019 by Chain Drug Review
Amneal Pharmaceuticals, Rob Stewart
Pharmacy, Supplier News

BRIDGEWATER, N.J. — Amneal Pharmaceuticals announced this week a comprehensive restructuring plan designed to reduce its cost base, further right size its organization and optimize its global manufacturing infrastructure. “Recently we initiated an in depth, company-wide review of our organizational structures, operational budgets, current and future capital projects, and existing capability and infrastructure alignments,” said
January 4, 2019 by Chain Drug Review
Amneal Pharmaceuticals, David Buchen, Inc., Robert Stewart
Supplier News

BRIDGEWATER, N.J. — Amneal Pharmaceuticals, Inc. has appointed David Buchen to the newly expanded role of senior vice president, chief legal officer and corporate secretary, reporting to oresident and chief executive officer Robert Stewart. Buchen will be responsible for leading Amneal’s global legal, Intellectual Property (IP) and corporate compliance functions, as well as aligning the company’s corporate legal strategies to
May 7, 2018 by Chain Drug Review
Amneal Pharmaceuticals, Amneal Pharmaceuticals LLC, Chintu Patel, Chirag Patel, Impax Laboratories, Inc., Paul Bisaro, Rob Stewar
Leading Headlines, Pharmacy, Supplier News

BRIDGEWATER, N.J. — Amneal Pharmaceuticals LLC and Impax Laboratories, Inc. announced that they have completed their business combination to form Amneal Pharmaceuticals, Inc. As a diversified company with a robust generics business, Amneal is now the 5th largest generics business in the United States, with a growing, high-margin specialty franchise. Shares of Impax ceased trading
May 2, 2018 by Chain Drug Review
Amneal Pharmaceuticals, Andy Boyer, Sabril, vigabatrin
Pharmacy, Supplier News

BRIDGEWATER, N.J. — Amneal Pharmaceuticals has launched vigabatrin for oral solution USP, a generic equivalent for Sabril. This is the second generic vigabatrin product currently on the market. Each carton contains fifty 500 mg packets. The finished dosage form is manufactured in the United States. “We are pleased to add this critical, specialty product to
March 14, 2018 by Chain Drug Review
Amneal Pharmaceuticals, erythromycin tablets, Gabitril, tiagabine hydrochloride tablets
Pharmacy, Supplier News

BRIDGEWATER, N.J. — Amneal Pharmaceuticals has launched two generic drugs, including first-to-market erythromycin tablets. The Amneal erythromycin product, in 250 mg and 500 mg strengths, is a therapeutic equivalent for the reference listed drug erythromycin tablets from Arbor Pharmaceuticals and is the only other immediate release oral tablet available. “Amneal is committed to increasing access to
February 1, 2018 by Bill Schiffner
Amneal Pharmaceuticals, isotretinoin capsules
Pharmacy, Supplier News

BRIDGEWATER, NJ — Amneal Pharmaceuticals has launched isotretinoin capsules, USP, an AB-rated generic for Accutane used to treat severe recalcitrant nodular acne in 10 mg, 20 mg, 30 mg and 40 mg. The product is available in cartons containing 3 blister packs of 10 capsules each and it began shipping today. Amneal’s generic received FDA approval













