January 22, 2018 by Chain Drug Review
Amneal Pharmaceuticals, budesonide capsules, generic version of Entocort EC
Pharmacy, Supplier News

BRIDGEWATER, N.J. — Amneal Pharmaceuticals has rolled out budesonide capsules 3 mg, a medication for Crohn’s disease. Amneal said its budesonide capsules are a generic version of Entocort EC from AstraZeneca plc. The generic product has begun shipping to wholesalers, distributors and direct to the trade, Amneal added. A steriod medication, budesonide reduces inflammation in the body and is
December 20, 2017 by Chain Drug Review
Allergan, Amneal Pharmaceuticals, Amneal-Impax merger, Brent Saunders, Chintu Patel, Chirag Patel, Impax Laboratories, Paul Bisaro, Robert Stewart, Wayne Swanton
Business, Supplier News

NEW YORK — Merger partners Amneal Pharmaceuticals LLC and Impax Laboratories Inc. have tabbed Robert Stewart, executive vice president and chief operating officer of Allergan plc, to lead the combined company. Plans call for Stewart to join Amneal as president effective Jan. 25. After Amneal and Impax finalize their merger, announced in mid-October, he will
October 17, 2017 by Chain Drug Review
Amneal Pharmaceuticals, Amneal Pharmaceuticals Inc., Amneal-Impax merger, Chintu Patel, Chirag Patel, Impax Laboratories, Paul Bisaro
Business, Leading Headlines, Pharmacy, Supplier News

NEW YORK — Amneal Pharmaceuticals LLC and Impax Laboratories Inc. plan to merge in an all-stock transaction. An Amneal-Impax merger would create the fifth-largest generics supplier by gross revenue in the United States. To be named Amneal Pharmaceuticals Inc., the new entity will also possess a growing, high-margin specialty drug franchise. Financial terms of the deal
August 9, 2017 by Chain Drug Review
Amneal Pharmaceuticals, generic version of Tamiflu, oseltamivir phosphate capsules
Pharmacy, Supplier News
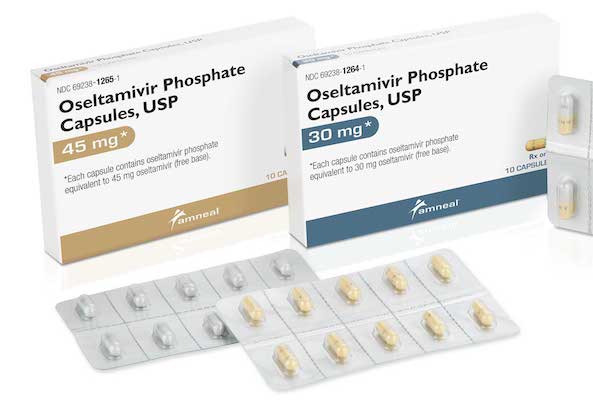
BRIDGEWATER, N.J. — Amneal Pharmaceuticals has launched oseltamivir phosphate capsules, an antiviral medication used to treat symptoms of influenza. Amneal said its oseltamivir phosphate product, a generic version of Tamiflu from Genentech, comes in dosages of 30 mg, 45 mg and 75 mg. Each strength is available in 10-count blister dose packs. Manufactured in Amneal’s recently expanded
April 28, 2017 by Chain Drug Review
Amneal Pharmaceuticals, Arnold and Marie Schwartz College of Pharmacy, Chintu Patel, John Pezzuto, LIU Pharmacy
Pharmacy, Supplier News

BROOKLYN, N.Y. — Amneal Pharmaceuticals co-chief executive officer Chintu Patel will be the featured keynote speaker at the commencement ceremony for Long Island University’s Arnold and Marie Schwartz College of Pharmacy and Health Sciences. The event will be held on May 7 at the Tilles Center for the Performing Arts on the LIU Post campus
April 12, 2017 by Chain Drug Review
Amneal Pharmaceuticals, generic version of Nasonex, Jim Luce, mometasone furoate nasal spray
Pharmacy, Supplier News
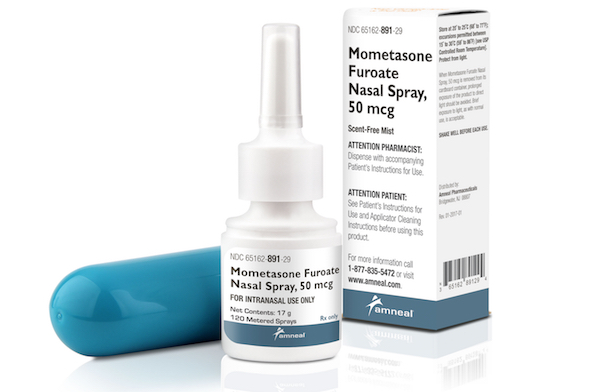
BRIDGEWATER, N.J. — Amneal Pharmaceuticals LLC has launched mometasone furoate nasal spray, which marks the company’s first pharmaceutical product in spray form. Amneal said its mometasone furoate product, a generic version of Nasonex, is available in 50 mcg/spray strength and packaged in a white, high-density, polyethylene bottle fitted with a white, metered-dose, manual spray pump
January 31, 2017 by Chain Drug Review
alosetron hydrochloride tablets, Amneal Pharmaceuticals, generic version of Lotronex, irritable bowel syndrome
Pharmacy, Supplier News

BRIDGEWATER, N.J. — Amneal Pharmaceuticals LLC has released alosetron hydrochloride tablets, a treatment for irritable bowel syndrome (IBS) in women. Amneal said its alosetron hydrochloride product, a generic version of Lotronex from Sebela Pharmaceuticals Inc., comes in 0.5-mg and 1-mg strengths and began shipping in 30-count bottles through wholesalers, distributors and directly to the trade
September 19, 2016 by Chain Drug Review
Amneal Pharmaceuticals, Chirag Patel, estradiol vaginal inserts, generic equivalent of Vagifem, women's health, Yuvafem
Pharmacy, Supplier News
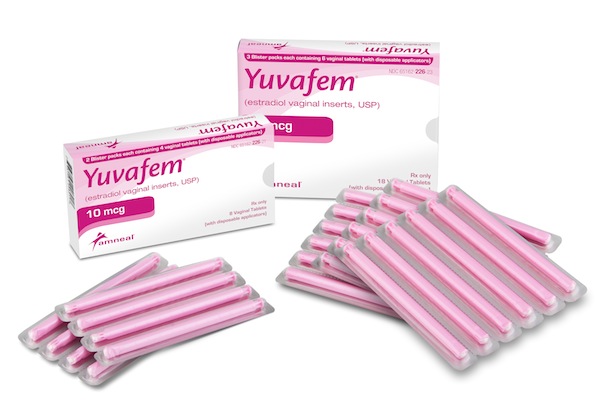
BRIDGEWATER, N.J. — Amneal Pharmaceuticals LLC next month plans to begin shipping Yuvafem 10-mcg estradiol vaginal inserts, a treatment for menopausal symptoms. Amneal said Monday that its product represents the first generic equivalent of Vagifem from Novo Nordisk. Yuvafem delivers the same low dose of vaginal estrogen in a preloaded, single-use, disposable applicator. Amneal said
September 9, 2016 by Chain Drug Review
Amneal Pharmaceuticals, birth control pill, Femynor, generic version of Ortho-Cyclen 28, oral contraceptive, women's health products
Pharmacy, Supplier News

BRIDGEWATER, N.J. — Amneal Pharmaceuticals LLC has expanded its portfolio of women’s health products with the release of Femynor, a birth control pill. Amneal said Femynor (norgestimate and ethinyl estradiol tablets, 0.25 mg/0.035 mg) is a generic version of Ortho-Cyclen 28 from Janssen Pharmaceuticals, a subsidiary of Johnson & Johnson. Femynor is sold in a
June 17, 2016 by Chain Drug Review
Amneal Pharmaceuticals, Chirag Patel, DIANA awards, generic drug manufacturer, generic pharmaceutical, HDA, Healthcare Distribution Alliance
Pharmacy, Supplier News

BRIDGEWATER, N.J. — Amneal Pharmaceuticals has been recognized as a top generic drug manufacturer by the Healthcare Distribution Alliance (HDA). Amneal said that this week it received the Distribution Industry Award for Notable Achievements in Healthcare (DIANA) for Generic Pharmaceutical Product Manufacturer, for the $100 million-plus sales to health care distributors category. HDA presented the
December 9, 2015 by Chain Drug Review
Amneal Pharmaceuticals, Jim Luce, lidocaine
Pharmacy, Supplier News

BRIDGEWATER, N.J — Amneal Pharmaceuticals LLC has begun shipping lidocaine 5% ointment to lead off the launch of a new lidocaine product line. Amneal said late Tuesday that its lidocaine 5% ointment is available in a 35.44-g tube with child-resistant cap and in a 50-g jar. The white, scent-free topical is now available via wholesalers,
October 23, 2015 by Chain Drug Review
Amneal Pharmaceuticals, biosimilars, Chirag Patel, generic drug, generic pharmaceuticals
Pharmacy, Supplier News

BRIDGEWATER, N.J. — Amneal Pharmaceuticals won the award for “Company of the Year, Americas” at the 2015 Global Generics and Biosimilars Awards held in Madrid, Spain. Amneal said late Thursday that it was the second straight year the company was recognized by Generics Bulletin, a global newsletter for the generic drug and biosimilars industries. In
August 25, 2015 by Chain Drug Review
Amneal Biosciences, Amneal Pharmaceuticals, Charles Lucarelli, Chirag Patel, generic drugs, specialty pharmaceuticals
Pharmacy, Supplier News

BRIDGEWATER, N.J. — Amneal Pharmaceuticals LLC has formed Amneal Biosciences, a new business unit. Amneal said Tuesday that the new subsidiary will focus exclusively on the commercialization of high-barrier-to-entry generic drugs and specialty pharmaceuticals — such as injectables, oncologics and biosimilars — health care providers worldwide. “Having a dedicated company focused on the unique needs and
August 18, 2015 by Chain Drug Review
Amneal Pharmaceuticals, aripiprazole, Arthur Bedrosian, generic versions of Abilify oral solution, Jim Luce, Lannett
Pharmacy, Supplier News

NEW YORK — Lannett Co. and Amneal Pharmaceuticals LLC have launched the first generic versions of Abilify oral solution (aripiprazole 1 mg/ml), Otsuka Pharmaceutical’s antidepressant drug. Lannett said Tuesday that its Silarx Pharmaceuticals subsidiary received Food and Drug Administration for its generic version of Abilify oral solution. “Our generic aripiprazole 1 mg/ml oral solution product













