August 31, 2016 by Chain Drug Review
benzodiazepines, FDA, Food and Drug Administration, opioid analgesic misuse, opioid analgesics, prescription opioid cough products, Robert Califf
Featured Articles, Leading Headlines, Pharmacy, Retail News

SILVER SPRING, Md. — Citing dangers from combined use, the Food and Drug Administration is requiring classwide changes to labeling for opioid analgesics, prescription opioid cough products and benzodiazepines, a group of central nervous system depressant drugs. The FDA said Wednesday that the changes mandate boxed warnings — the agency’s strongest warning — and patient
July 8, 2016 by Chain Drug Review
Alex Gourlay, Brad Fluegel, FDA, Food and Drug Administration, National Institutes of Health, next-generation sequencing, Precision Medicine Initiative, Precision Medicine Initiative Cohort Program, Robert Califf, Walgreens, Walgreens Boots Alliance
Leading Headlines, Pharmacy, Retail News

DEERFIELD, Ill. — Walgreens plans to participate in the U.S. Precision Medicine Initiative Cohort Program, part of a sweeping federal medical research initiative to help develop individualized care based on a person’s genomic makeup. Walgreens said it’s taking part in the PMI Cohort Program via an initial $20 million grant awarded to The Scripps Research
July 8, 2016 by Chain Drug Review
acne treatment, adapalene gel, Differin Gel, FDA, Food and Drug Administration, Galderma, Lesley Furlong, Miles Harrison, Philip Brown
Pharmacy, Supplier News

FORT WORTH, Texas — Galderma Laboratories L.P. has gained Food and Drug Administration approval for Differin Gel, an over-the-counter treatment for acne. Galderma said Friday that Differin Gel (adapalene gel 0.1%) is the first and only OTC acne product with a full prescription-strength retinoid, and the product represents the first new, FDA-approved active ingredient to
May 20, 2016 by Chain Drug Review
Chip Davis, EAR, Expedited Agency Review, FDA, Food and Drug Administration, generic drug labeling, generic drug manufacturers, generic drugs, Generic Pharmaceutical Association, GPhA
Featured Articles, Leading Headlines, Pharmacy, Supplier News
WASHINGTON — The Food and Drug Administration is again postponing the release of a final rule that would change generic drug labeling requirements. In a Federal Register notice this week, the FDA indicated that it aims to implement the final rule in April 2017, though many industry stakeholders had expected the agency to do so
May 19, 2016 by John Schultz and Chain Drug Review
FDA’s Office of Generic Drugs, Food and Drug Administration, generic drug approval process, generic drugs, Kathleen Uhl
Featured Articles, Leading Headlines, Pharmacy, Supplier News
WASHINGTON — Amid calls for a quicker approval process, the Food and Drug Administration last year cleared for market the most generic drug products in its history. The FDA said it approved or issued tentative approvals for more than 700 generic drugs in 2015. The announcement may help to blunt criticism from some federal legislators
April 6, 2016 by Chain Drug Review
biosimilar, Celltrion, FDA, Food and Drug Administration, Hospira, Inflectra, Jane Woodcock, Janssen Biotech, Jenny Alltoft, Pfizer, Remicade, Salomon Azoulay
Leading Headlines, Pharmacy, Supplier News

WASHINGTON — Just over a year after approving its first biosimilar product, the Food and Drug Administration has approved its second, Celltrion’s Inflectra (biosimilar infliximab). Inflectra is a biosimilar to Janssen Biotech’s Remicade (infliximab), originally licensed in 1998. Inflectra is indicated for reducing signs and symptoms in patients with rheumatoid arthritis, adult ulcerative colitis, plaque
February 24, 2016 by Chain Drug Review
FDA, FDA commissioner, Food and Drug Administration, Margaret Hamburg, Robert Califf, Stephen Ostroff, Sylvia Burwell
Leading Headlines, Pharmacy, Retail News

WASHINGTON — The U.S. Senate overwhelmingly confirmed Dr. Robert Califf as commissioner of the Food and Drug Administration. Califf, who was deputy commissioner for medical products and tobacco, was nominated as FDA commissioner by President Barack Obama in mid-September. No significant opposition was expected for his confirmation hearing on Tuesday, as the Senate voted 89-4
February 4, 2016 by Chain Drug Review
community pharmacies, Food and Drug Administration, House Committee on Oversight and Government Reform, Janet Woodcock, Mark Merritt, National Community Pharmacists Association, PBM industry, Pharmaceutical Care Management Association, pharmacy benefit management, video
Leading Headlines, Pharmacy, Retail News, Videos

ALEXANDRIA, Va. — In comments to Congress, the National Community Pharmacists Association called for more oversight of pharmacy benefit management (PBM) companies. NCPA said payers, pharmacy patients and pharmacists could see “tangible benefits” from increased transparency into PBM business practices and potential conflicts of interests. The association submitted its comments to the House Committee on
December 21, 2015 by Chain Drug Review
American Herbal Products Association, Consumer Healthcare Products Association, Council for Responsible Nutrition, dietary supplement, FDA, Food and Drug Administration, Natural Products Association, ODSP, Office of Dietary Supplement Programs, United Natural Products Alliance
Leading Headlines, Supplier News

WASHINGTON — The Food and Drug Administration has established the Office of Dietary Supplement Programs (ODSP), raising the program from its previous status as a division under the Office of Nutrition Labeling and Dietary Supplements. The FDA said Monday that it’s also in the process of identifying permanent leadership for ODSP. Meanwhile, Bob Durkin will
October 29, 2015 by Chain Drug Review
biological products, biosimilar, biosimilar naming convention, Federal Trade Commission, Food and Drug Administration
Leading Headlines, Pharmacy, Supplier News

WASHINGTON — The staff of the Federal Trade Commission has submitted comment for the Food and Drug Administration’s draft guidance on nonproprietary names for biological products. The FTC said Wednesday that its staff comment expressed concern that the FDA draft guidance on biosimilar naming may hinder competition and, as a result, recommended that the agency
September 28, 2015 by Brian Bossetta and Chain Drug Review
Food and Drug Administration, Robert Califf, Stephen Ostroff
2015, Issue 09-28-2015, Issues, News
WASHINGTON — The Food and Drug Administration’s current deputy commissioner for medical products and tobacco is likely to become the organization’s next commissioner. Dr. Robert Califf, a cardiologist and clinical researcher long affiliated with Duke University, was nominated by President Obama earlier this month to head up the FDA. Though Califf must face Senate confirmation
September 16, 2015 by Chain Drug Review
Barack Obama, FDA commissioner, Food and Drug Administration, Margaret Hamburg, Robert Califf, Stephen Ostroff
Leading Headlines, Pharmacy, Retail News

WASHINGTON — The Food and Drug Administration’s current deputy commissioner for medical products and tobacco is likely to become the organization’s next commissioner. Dr. Robert Califf, a cardiologist and clinical researcher long affiliated with Duke University, on Tuesday was nominated as FDA commissioner by President Barack Obama. On Wednesday, his nomination was sent to the
August 31, 2015 by Chain Drug Review
Academy of Managed Care Pharmacy, AMCP, Bertrand Liang, biologic drugs, biologic products, Biologics Prescribers Collaborative, biosimilar product names, biosimilars, Biosimilars Forum, Center for Biologics Evaluation and Research, Center for Drug Evaluation and Research, Edith Rosato, Food and Drug Administration, GPhA, Janet Woodcock, Karen Midthun, The Biosimilars Council
Featured Articles, Leading Headlines, Pharmacy, Supplier News

WASHINGTON — Early feedback on the Food and Drug Administration’s proposal on naming conventions for biosimilars shows a mixed response. Late late week, the FDA published draft guidance recommending that reference biologic products and biosimilars have nonproprietary names that share a core drug substance name and an FDA-designated suffix unique for each product. For interchangeable
August 19, 2015 by Chain Drug Review
Addyi, CDER, Center for Drug Evaluation and Research, Cindy Whitehead, female sexual dysfunction, Food and Drug Administration, HSDD, hypoactive sexual desire disorder, Janet Woodcock, Sprout Pharmaceuticals
Pharmacy, Supplier News
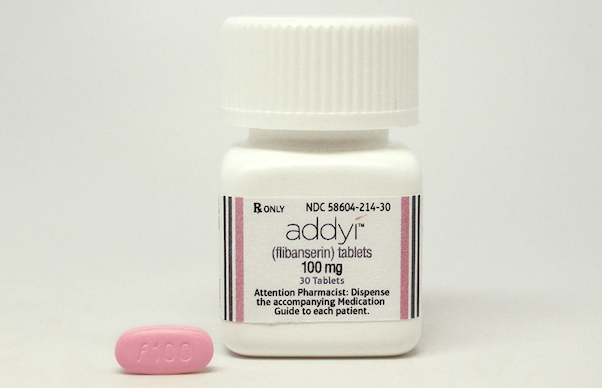
RALEIGH, N.C. — Sprout Pharmaceuticals Inc. has gained Food and Drug Administration clearance to market Addyi, which the company calls the first FDA-approved medication for hypoactive sexual desire disorder (HSDD) in women. Sprout said late Tuesday that Addyi (flibanserin, 100 mg) is slated to be released by Oct. 17. “It has been a remarkable journey to













