July 7, 2015 by Chain Drug Review and Chain Drug Review
Center for Drug Evaluation and Research, cystic fibrosis, Cystic Fibrosis Foundation, Food and Drug Administration, Jeffrey Leiden, John Jenkins, lumacaftor/ivacaftor, Office of New Drugs, Orkambi, Robert Beall, Vertex Pharmaceuticals
Pharmacy, Supplier News
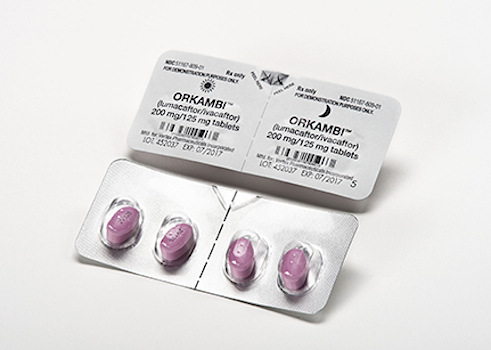
BOSTON — The approval of Vertex Pharmaceuticals Inc.’s Orkambi (lumacaftor/ivacaftor) is being hailed as a big stride forward in the treatment of cystic fibrosis. The Food and Drug Administration announced its approval of the drug just before the holiday weekend. Vertex noted that the medication is the first to treat the underlying cause of cystic fibrosis in
July 1, 2015 by Chain Drug Review
Drug Supply Chain Security Act, DSCSA, Food and Drug Administration, National Association of Chain Drug Stores, pharmacies
Featured Articles, Leading Headlines, Retail News

WASHINGTON — Pharmacies have a four-month extension — until November 1 — to comply with the full scope of new dispenser tracing requirements under the Drug Supply Chain Security Act (DSCSA). The Food and Drug Administration issued a guidance yesterday saying it would not take enforcement action until November against a pharmacy that receives any
June 24, 2015 by Chain Drug Review
American Pharmacists Association, APhA, B. Douglas Hoey, Drug Supply Chain Security Act, DSCSA, Food and Drug Administration, NASPA, National Alliance of State Pharmacy Associations, National Community Pharmacists Association, NCPA, pharmaceutical supply chain, Rebecca Snead, Thomas Menighan
Leading Headlines, Pharmacy, Retail News

ALEXANDRIA, Va. — The National Community Pharmacists Association (NCPA), American Pharmacists Association (APhA) and National Alliance of State Pharmacy Associations (NASPA) have asked the Food and Drug Administration to delay enforcement of a July 1 product tracing deadline for dispensers of pharmaceuticals. In a letter this week to the FDA’s Office of Compliance, the three
June 16, 2015 by Chain Drug Review and Chain Drug Review
FDA, FDA’s Center for Food Safety and Applied Nutrition, Food and Drug Administration, food manufacturers, partially hydrogenated oils, PHOs, processed foods, Stephen Ostroff, Susan Mayne, trans fat
Leading Headlines, Supplier News

WASHINGTON — To eliminate trans fat in processed foods, the Food and Drug Administration has called on food manufacturers to remove partially hydrogenated oils (PHOs) from products over the next three years. The FDA said Tuesday that, following “a thorough review of the scientific evidence,” it has concluded that PHOs — the primary dietary source
April 21, 2015 by Chain Drug Review and Chain Drug Review
AAHP, American Association of Homeopathic Pharmacists, Food and Drug Administration, homeopathic medicines, homeopathic pharmacists, Mark Land
Retail News
SILVER SPRING, Md. — The Food and Drug Administration’s regulatory policy for homeopathic medicines has worked effectively for the last 25 years to protect the public health and provide consumer access to safe homeopathic medicines, according to the American Association of Homeopathic Pharmacists (AAHP). AAHP, which represents 90% of the U.S. homeopathic market, made its
March 6, 2015 by John Schultz and Chain Drug Review
biologics, biosimilar, filgrastim, Food and Drug Administration, Margaret Hamburg, Sandoz, Zarxio
News, Pharmacy, Retail News

WASHINGTON — Zarxio (filgrastim), a cancer treatment from Sandoz Inc., has become the first biosimilar product approved in the United States. The Food and Drug Administration announced its approval of Zarxio, a biosimilar to Neupogen, an oncology product from Amgen Inc., on Friday. The move is being hailed as a watershed event that paves the
February 16, 2015 by Mark Baumgartner and Chain Drug Review
Food and Drug Administration, Francis Collins, Generic Pharmaceutical Association, Margaret Hamburg, National Institutes of Health, Ralph Neas, Stephen Ostroff
2015, Issue 02-16-2015, News, Pharmacy
WASHINGTON — Dr. Margaret Hamburg, commissioner of the Food and Drug Administration, will step aside next month after nearly six years at the helm of the agency overseeing many of the nation’s most contentious public policy issues and products representing about 20% of every dollar spent by consumers. Hamburg, 59, said her decision to step
February 5, 2015 by Chain Drug Review
FDA, FDA commissioner, Food and Drug Administration, Margaret Hamburg, Stephen Ostroff
Pharmacy, Retail News
Margaret Hamburg WASHINGTON — Margaret Hamburg reportedly is stepping down as commissioner of the Food and Drug Administration. Published reports said Thursday that the 59-year-old Hamburg is slated to leave the FDA commissioner post at the end of March and that the Obama administration plans to make a formal announcement on Friday. Plans call for
January 19, 2015 by John Schultz and Chain Drug Review
Drug Supply Chain Security Act, DSCSA, FDA, Food and Drug Administration, Food Drug and Cosmetic Act, HDMA, Healthcare Distribution Management Association, John Parker, pharmaceutical supply chain, prescription drugs, product tracing rules
2015, Issue 01-19-2015, News, Pharmacy
WASHINGTON — The Healthcare Distribution Management Association (HDMA) is praising the Food and Drug Administration for its flexibility pertaining to new product tracing rules, specifically on the FDA’s recently released guidance titled “Product Tracing Requirements — Compliance Policy.” The association had sent letters to the regulatory agency in November and then again last month in
January 19, 2015 by John Schultz and Chain Drug Review
biosimilar, cancer treatment, Center for Drug Evaluation and Research, FDA, Food and Drug Administration, Generic Pharmaceutical Association, Janet Woodcock, Johnson & Johnson, Merck, Neupogen, Neupogen biosimilar, Ralph Neas, Remicade, rheumatoid arthritis drug, Sandoz, Zarzio
2015, Issue 01-19-2015, News, Pharmacy
WASHINGTON — A Food and Drug Administration panel has recommended that the agency approve what could become the country’s first biosimilar. The panel unanimously urged approval of Sandoz’s filgrastin (Neupogen), a cancer treatment. “This encouraging step forward means that it is very likely now only a question of when, rather than if, filgrastin will be available






