July 29, 2019 by Chain Drug Review
Heather Bresch, Ken Parks, mylan, Pfizer, Rajiv Malik, Robert Coury, Upjohn
Business, Featured Articles, Leading Headlines, Pharmacy, Supplier News

NEW YORK— Pfizer and Mylan announced Monday a definitive agreement to combine Mylan with Upjohn, Pfizer’s off-patent branded and generic established medicines business, creating a new global pharmaceutical company. Under the terms of the agreement, which is structured as an all-stock, Reverse Morris Trust transaction, each Mylan share would be converted into one share of
August 13, 2018 by Chain Drug Review
Adcirca, Heather Bresch, Mylan N.V., tadalafil tablets
Pharmacy, Supplier News

HERTFORDSHIRE, England and PITTSBURGH — Mylan N.V. announced the U.S. launch of tadalafil tablets USP, 20 mg, the first generic version of the reference listed drug, Eli Lilly and company’s Adcirca. Mylan Pharmaceuticals received final approval from the U.S. Food and Drug Administration (FDA) for its Abbreviated New Drug Application (ANDA) and was awarded 180 days of
June 11, 2018 by Chain Drug Review
Americares, Healthcare Distribution Alliance (HDA), Heather Bresch, John Gray, Michael Nyenhuis, mylan
Pharmacy, Supplier News

STAMFORD, Conn. – Americares, in partnership with the Healthcare Distribution Alliance (HDA), has presented its annual Power of Partnership Award to Mylan, in recognition of its outstanding commitment to increasing access to health care around the world. Mylan is among Americares most dedicated and longstanding supporters, providing pharmaceutical products for the organization’s health programs for people affected
May 14, 2018 by Chain Drug Review
Heather Bresch, Mylan Pharmaceuticals, STEM, West Virginia University
Leading Headlines, Supplier News

MORGANTOWN, W.Va. — Global pharmaceutical company Mylan and West Virginia University (WVU) today announced a 10-year collaboration, supported by a $5 million charitable contribution from Mylan, to develop and implement a program that will expose children across West Virginia to STEM (Science, Technology, Engineering and Math) and challenge traditional thinking about how STEM skills can be
April 3, 2018 by Bill Schiffner
Heather Bresch, HIV Treatment, mylan, Symfi Triple Combo
Pharmacy, Supplier News

HERTFORDSHIRE, England and PITTSBURGH — Global pharmaceutical company Mylan N.V. recently announced that it will introduce in the U.S. a third cost-saving HIV combination. The U.S. Food and Drug Administration (FDA) approved Symfi (efavirenz, lamivudine and tenofovir disoproxil fumarate) 600 mg/300 mg/300 mg tablets, a once-daily, single-tablet regimen (STR), indicated as a complete regimen for the treatment
December 1, 2017 by Chain Drug Review
Arun Chandavarkar, Biocon, Genentech, Heather Bresch, Herceptin, Herceptin biosimilar, mylan, Ogivri, trastuzumab, trastuzumab-dkst
Pharmacy, Supplier News

PITTSBURGH and BENGALURU, India — Mylan N.V. has received Food and Drug Administration approval for Ogivri (trastuzumab-dkst), a biosimilar oncology drug co-developed with Biocon Ltd. Mylan said Friday that Ogivri is a biosimilar of Herceptin (trastuzumab) from Genentech, indicated for the treatment of HER2-overexpressing breast cancer and metastatic stomach cancer (gastric or gastroesophageal junction adenocarcinoma).
August 18, 2017 by Chain Drug Review
epinephrine auto-injectors, EpiPen, EpiPen Jr, Heather Bresch, Medicaid Drug Rebate Program, mylan
Pharmacy, Supplier News
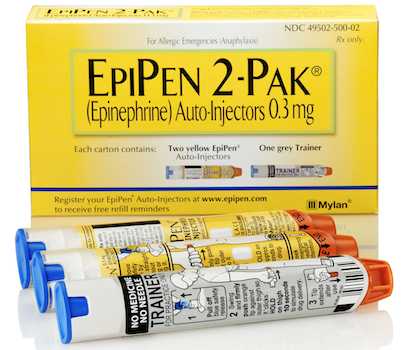
PITTSBURGH — Mylan Inc. and Mylan Specialty L.P. have entered an agreement with the U.S. Department of Justice finalizing the $465 million Medicaid drug rebate settlement last fall. Mylan said late Thursday that the settlement, announced Oct. 7, resolves claims over the classification of the EpiPen and EpiPen Jr epinephrine auto-injectors in the Medicaid Drug
March 30, 2017 by Chain Drug Review
Cold-EEZE, Heather Bresch, mylan
Business, Pharmacy, Supplier News

PITTSBURGH — Furthering its expansion in the consumer health care arena, Mylan has closed its acquisition of the Cold-EEZE over-the-counter cold remedy line from ProPhase Labs Inc. The transaction, announced in January, gives Mylan all global rights and assets relating to the Cold-EEZE brand, including all U.S. businesses and U.S. and international trademarks. Cold-EEZE’s broad
February 14, 2017 by Chain Drug Review
AAM, Apotex, Association for Accessible Medicines, biosimilars, Chip Davis, generic drugs, Generic Pharmaceutical Association, GPhA, GPhA Annual Meeting, Heather Bresch, Jeff Watson, Lupin Pharmaceuticals, mylan, Paul McGarty, Peter Goldschmidt, Sandoz
Featured Articles, Leading Headlines, Pharmacy, Supplier News

WASHINGTON — The Generic Pharmaceutical Association (GPhA), which represents manufacturers of generic drugs and biosimilars, has changed its name to the Association for Accessible Medicines (AAM). The association said Tuesday that the new name better reflects its mission: to make medications more accessible to the people who need them. As GPhA, the trade group had steadfastly
December 30, 2016 by Chain Drug Review
generic version of Concerta, Heather Bresch, medication for attention deficit hyperactivity disorder, methylphenidate hydrochloride extended-release tablets, mylan
Pharmacy, Supplier News

PITTSBURGH — Mylan N.V. has launched methylphenidate hydrochloride extended-release tablets, a medication for attention deficit hyperactivity disorder (ADHD), in the United States. Mylan said its product, a generic version of Concerta tablets from Janssen Pharmaceuticals Inc., comes in dosages of 18 mg, 27 mg, 36 mg and 54 mg. “The launch of generic Concerta tablets
December 20, 2016 by Chain Drug Review
epinephrine auto-injector, generic EpiPen, Heather Bresch, mylan
Pharmacy, Supplier News
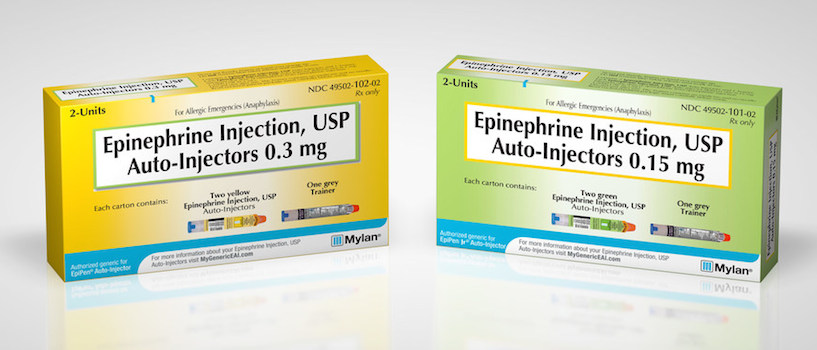
PITTSBURGH — This week, Mylan N.V. began making an authorized generic of its EpiPen epinephrine auto-injector available to pharmacies. Mylan said its generic epinephrine auto-injector, announced in late August, carries a wholesale acquisition cost (WAC) of $300 per two-pack carton, more than 50% lower than the WAC of EpiPen 2-Pak Auto-Injectors. The generic EpiPen, which
September 22, 2016 by Chain Drug Review
EpiPen, Heather Bresch, House Committee on Oversight and Government Reform, mylan, video
Leading Headlines, Videos

Mylan CEO Heather Bresch gave testimony at a hearing Wednesday afternoon by the House Committee on Oversight and Government Reform over the sharp price hike for the EpiPen epinephrine auto-injector since the company acquired the product in 2007. The EpiPen is used for treating anaphylaxis, a potentially life-threatening allergic reaction. Committee chairman Jason Chaffetz (R.,
August 29, 2016 by Chain Drug Review
anaphylaxis, Chuck Grassley, epinephrine auto-injector, EpiPen, Heather Bresch, mylan
Leading Headlines, Pharmacy, Supplier News
PITTSBURGH — In what chief executive officer Heather Bresch called “an extraordinary commercial response,” Mylan N.V. plans to release a generic version of its EpiPen epinephrine auto-injector for anaphylaxis, answering public demands to lower the cost of the potentially life-saving product. Mylan said Monday that its U.S. subsidiary expects to roll out the generic EpiPen
May 13, 2016 by Chain Drug Review
dermatology, generic topical products, Heather Bresch, mylan, Rajiv Malik, Renaissance Acquisition Holdings
Pharmacy, Supplier News

PITTSBURGH — Mylan N.V. has entered an agreement to buy the nonsterile, topicals-focused specialty and generics business of Renaissance Acquisition Holdings LLC for $950 million in cash. Mylan said Friday that the Renaissance business, which has about 1,200 employees and posted sales of $370 million in 2015, will bring 25 branded and generic topical products,













