February 16, 2017 by Chain Drug Review
butalbital acetaminophen tablets, generic version of Bupap, Mayne Pharma, migraine medication, Mikart, Scott Richards
Pharmacy, Supplier News

GREENVILLE, N.C. — Mayne Pharma Inc. has launched butalbital acetaminophen tablets 50 mg/300 mg, a migraine medication, in the United States. Mayne said Thursday that it will distribute the product, a generic version of Bupap tablets (butalbital acetaminophen 50 mg/300 mg) from Valeant Pharmaceuticals International, on behalf of its partner Mikart Inc., the developer and
January 12, 2017 by Chain Drug Review
Doryx, Fabior Foam, GlaxoSmithKline, Mayne Pharma, Mayne Pharma Specialty Brands, Scott Richards, Sorilux Foam
Pharmacy, Supplier News

GREENVILLE, N.C. — Mayne Pharma Inc. has rolled out Fabior Foam 0.1%, an acne treatment, and Sorilux Foam 0.005%, a psoriasis treatment, in the United States. Launched by the Mayne Pharma Specialty Brands division, Fabior and Sorilux foam previously were marketed by GlaxoSmithKline. Mayne acquired the two products in August 2016. Mayne said Fabior and
January 2, 2017 by Geoff Walden and Chain Drug Review
Aurobindo Pharma USA, Citron Pharma, generic drug manufacturers, George Jepsen, Heritage Pharmaceuticals, Jason Malek, Jeffrey Glazer, Mayne Pharma, Mylan Pharmaceuticals, Teva Pharmaceuticals USA
2017, Issue 01-02-2017, Issues, News
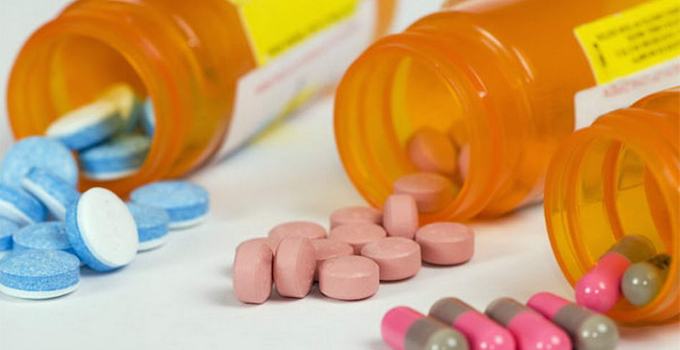
HARTFORD, Conn. — Twenty states have accused six generic drug manufacturers of price fixing. The states’ attorneys general also said their lawsuit, filed in federal court here, may be just the start of a much broader legal action. The suit charges that the drug makers, led by New Jersey-based Heritage Pharmaceuticals, conspired to artificially elevate
August 3, 2016 by Chain Drug Review
Allergan, generic drug companies, Mayne Pharma, Stefan Cross, Teva, U.S. generic market
Business, Pharmacy, Supplier News

GREENVILLE, N.C. — Mayne Pharma has completed its purchase of a package of generic drug products from Teva Pharmaceutical Industries Ltd. and Allergan plc. Mayne said Wednesday that under the $652 million deal, announced in late June, it takes ownership of 37 approved and five filed generic pharmaceutical products. The transaction was the largest generics
May 24, 2016 by Chain Drug Review
doxycycline hyclate delayed-release tablets, generic version of Doryx, Mayne Pharma, mylan
Pharmacy, Supplier News

PITTSBURGH — Mylan N.V. has launched doxycycline hyclate delayed-release tablets (200 mg), an antibiotic, in the United States. Mylan said its doxycycline product is a generic version of Doryx 200-mg delayed-release tablets from Mayne Pharma. A tetracycline-class antimicrobial, doxycycline hyclate DR tablets are a indicated as adjunctive therapy for severe acne. U.S. sales of 200-mg doxycycline






