October 27, 2022 by Chain Drug Review
Merck
Pharmacy, Supplier News

RAHWAY, N.J.— Merck announced that the Merck board of directors has unanimously elected Robert Davis to serve as chairman of the board, effective Dec. 1, 2022. He will succeed Kenneth Frazier, who plans to retire on Nov. 30, 2022, after a long-tenured career at Merck that began in 1992. Frazier led the company for 10
June 22, 2022 by Chain Drug Review
Merck
Pharmacy

RAHWAY, N.J. — Merck announced that Chirfi Guindo will lead Human Health Marketing as chief marketing officer for Merck Human Health, and will join Merck’s Executive Team, effective July 1, 2022. Guindo will report directly to Robert M. Davis, chief executive officer and president, Merck. As previously announced, Jannie Oosthuizen leads Merck Human Health U.S., and Joe
March 1, 2022 by Chain Drug Review
Dr. Julie Gerberding, Merck
Leading Headlines, Pharmacy, Supplier News

KENILWORTH, N.J. — Merck announced that Dr. Julie Gerberding, chief patient officer and executive vice president, population health and sustainability, will be retiring from Merck in May 2022. Earlier today, the Foundation for the National Institutes of Health (FNIH) announced that Dr. Gerberding will become CEO of the FNIH on May 16. “Julie’s accomplishments during
February 16, 2022 by Chain Drug Review
Arpa Garay, Deepak Khanna, Jannie Oosthuizen, Merck
Leading Headlines, Pharmacy

KENILWORTH, N.J. — Merck has announced new leadership for its Human Health business. Arpa Garay will lead Human Health Global Marketing, Jannie Oosthuizen will take the reins of Human Health U.S., and Merck is recruiting externally to fill the Human Health International role. In the interim, Deepak Khanna will lead Human Health International. These three positions will
December 23, 2021 by Chain Drug Review
Merck, molnupiravir
Leading Headlines, Pharmacy

WASHINGTON — The Food and Drug Administration on Thursday authorized Merck’s COVID-19 pill for certain adults. The authorization for molnupiravir is limited to adults over 18 who have a high risk of severe illness and “for whom alternative FDA-authorized treatment options are not accessible or medically appropriate,” the FDA said in a press release Thursday.
November 9, 2021 by Chain Drug Review
Merck, Ridgeback Biotherapeutics
Leading Headlines, Pharmacy

KENILWORTH, N.J. — Merck and Ridgeback Biotherapeutics announced Tuesday that the United States government will exercise two of its options to purchase a total of 1.4 million additional courses of molnupiravir, an investigational oral antiviral medicine, if the medicine is granted Emergency Use Authorization (EUA) or approval by the U.S. Food and Drug Administration (FDA),
October 11, 2021 by Chain Drug Review
COVID-19 pill, Merck
Leading Headlines, Pharmacy

NEW YORK — Drug maker Merck asked U.S. regulators on Monday to authorize its pill against COVID-19 in what would add an entirely new and easy-to-use weapon to the world’s arsenal against the pandemic. If approved by the FDA, a decision that could come in a few of weeks, it would be the first pill
October 1, 2021 by Chain Drug Review
COVID-19 pill, Merck, Ridgeback Biotherapeutics
Leading Headlines, Pharmacy, Supplier News

KENILWORTH, N.J. —Merck announced Friday that it has developed an experimental COVID-19 pill that is said to reduced hospitalizations and deaths by half in people recently infected with the coronavirus. The company said that it would soon ask health officials in the U.S. and around the world to authorize its use. If cleared, Merck’s drug
September 30, 2021 by Chain Drug Review
Acceleron Pharma, Merck
Leading Headlines, Pharmacy

KENILWORTH, N.J. — Merck and Acceleron Pharma announced on Thursday that the companies have entered into a definitive agreement under which Merck, through a subsidiary, will acquire Acceleron for $180 per share in cash for an approximate total equity value of $11.5 billion. Acceleron is focused on harnessing the power of the transforming growth factor
July 29, 2021 by Chain Drug Review
Cristal Downing, Merck
Leading Headlines, Pharmacy

KENILWORTH, N.J. — Merck announced that Cristal Downing has been appointed chief communications & public affairs officer, a newly created role, effective August 16, 2021. Downing will become a member of Merck’s Executive Team and lead the development, advancement and execution of the company’s communications and public affairs strategy. “Cristal is a transformational leader with
March 24, 2021 by Chain Drug Review
Caroline Litchfield, Merck
Business, Leading Headlines, Pharmacy, Supplier News

KENILWORTH, N.J. — Merck announced Wednesday that Caroline Litchfield has been appointed executive vice president and chief financial officer (CFO), effective April 1, 2021. Litchfield succeeds Robert Davis; as previously announced (link), Davis, Merck’s current CFO, will become president of Merck, effective April 1, 2021, and will become chief executive officer on July 1, 2021.
March 17, 2021 by Chain Drug Review
Frank Clyburn, Merck, Michael Nally
Business, Leading Headlines, Supplier News

KENILWORTH, N.J. — Merck announced that Michael Nally, chief marketing officer, Human Health, will leave the company at the end of March, and Frank Clyburn, chief commercial officer, Human Health, will become president, Human Health and lead all Human Health commercial and marketing for the company. Nally is leaving Merck for a leadership opportunity with
March 15, 2021 by Chain Drug Review
Gilead Sciences, HIV Treatment, Merck
Leading Headlines, Pharmacy

KENILWORTH, N.J. – Gilead Sciences and Merck today announced that they have entered into an agreement to co-develop and co-commercialize long-acting treatments in HIV that combine Gilead’s investigational capsid inhibitor, lenacapavir, and Merck’s investigational nucleoside reverse transcriptase translocation inhibitor, islatravir, into a two-drug regimen with the potential to provide new, meaningful treatment options for people
March 2, 2021 by Chain Drug Review
Johnson & Johnson vaccine, Merck, President Biden
Featured Articles, Leading Headlines, Pharmacy
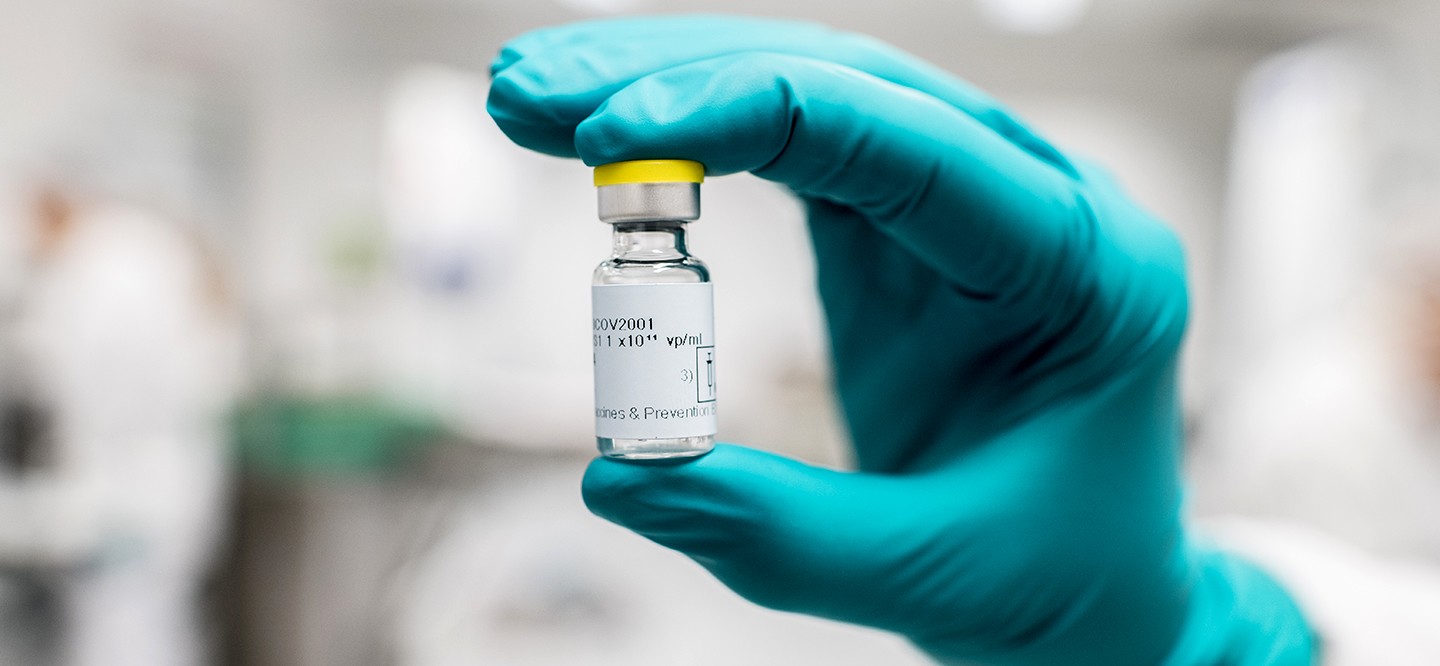
WASHINGTON — Merck will help produce rival Johnson & Johnson’s newly approved coronavirus vaccine in an effort to expand supply more quickly, the White House reported Tuesday. This announcement comes as the White House looks to ramp up the production of the single-dose vaccine. Officials have said J&J faced unexpected production issues with its vaccine




