January 4, 2021 by Bill Schiffner
Aurobindo Pharma Limited, clorazepate dipotassium tablets, mylan, Paul McMahon
Leading Headlines, Pharmacy, Supplier News

EAST WINDSOR, N.J. – Aurobindo Pharma Limited has acquired clorazepate dipotassium tablets 3.75mg, 7.5mg and 15mg from Mylan Pharmaceuticals, a Viatris Co.. Mylan has agreed to transfer the FDA-approved ANDA for each of these products to Aurobindo Pharma USA, Inc. and Aurobindo will begin to market, distribute, and sell these products as of January 1,
October 30, 2020 by Chain Drug Review
mylan, Pfizer, Upjohn
Business, Supplier News

Hertfordshire, England – Mylan and Pfizer announced that the U.S. Federal Trade Commission accepted a proposed consent order, which concludes the FTC’s review of the proposed combination of Mylan and Pfizer’s Upjohn Business. The parties have now obtained all required antitrust clearances for the proposed transaction. The combination will be effected through a Reverse Morris
August 31, 2020 by Chain Drug Review
Biocon Biologics India Ltd, diabetes, mylan, Mylan CEO Heather Bresch, Mylan president Rajiv Malik
Leading Headlines, Supplier News

PITTSBURGH— Mylan N.V. and Biocon Biologics India Ltd., a subsidiary of Biocon Ltd., announced on Monday the U.S. launch of Semglee (insulin glargine injection) in vial and pre-filled pen presentations, approved to help control high blood sugar in adult and pediatric patients with type 1 diabetes and adults with type 2 diabetes. It is not
July 10, 2020 by Chain Drug Review
mylan, Robert Coury, Upjohn, Viatris
Leading Headlines, Pharmacy

HERTFORDSHIRE, England— Mylan and Upjohn, a division of Pfizer, unveiled on Thursday the logo and branding for Viatris, the new company that will be formed by combining Mylan and Upjohn. Viatris will benefit from Mylan and Upjohn’s trusted global offerings and legacy of meeting patient needs around the world. The new logo and branding is
July 1, 2020 by Chain Drug Review
mylan, Upjohn, Viatris
Business, Leading Headlines, Pharmacy

HERTFORDSHIRE, England – Mylan announced that its shareholders overwhelmingly voted to approve the proposed transaction combining Mylan and Upjohn, a division of Pfizer, at the company’s extraordinary general meeting of shareholders. Approximately 99.6% of votes cast were voted in favor of the combination. Mylan executive chairman and future Viatris executive chairman Robert Coury said: “The
November 14, 2019 by Chain Drug Review
mylan, Pfzer, Robert Coury, Viatris
Pharmacy, Supplier News

NEW YORK — Mylan and Pfizer announced earlier this week that the name of the new company to be formed by the planned combination of Mylan and Upjohn, a division of Pfizer, will be Viatris (pronounced ‘viǝ-trīs). Deriving its name from Latin, Viatris embodies the new company’s goal of providing a path—“VIA”—to three—“TRIS”—core goals: expanding
July 29, 2019 by Chain Drug Review
Heather Bresch, Ken Parks, mylan, Pfizer, Rajiv Malik, Robert Coury, Upjohn
Business, Featured Articles, Leading Headlines, Pharmacy, Supplier News

NEW YORK— Pfizer and Mylan announced Monday a definitive agreement to combine Mylan with Upjohn, Pfizer’s off-patent branded and generic established medicines business, creating a new global pharmaceutical company. Under the terms of the agreement, which is structured as an all-stock, Reverse Morris Trust transaction, each Mylan share would be converted into one share of
June 28, 2018 by Chain Drug Review
biosimilar, Enbrel, Lupin, mylan, Rajiv Malik, Vinita Gupta
Pharmacy, Supplier News

MUMBAI, India and HERTFORDSHIRE, England and PITTSBURGH — Pharma major Lupin and Mylan announced that the two companies will partner to commercialize a biosimilar to Enbrel (etanercept). Through the partnership agreement, Mylan will commercialize Lupin’s proposed etanercept biosimilar in Europe, Australia, New Zealand, Latin America, Africa and most markets throughout Asia. Enbrel is a TNF-inhibitor indicated to
June 11, 2018 by Chain Drug Review
Americares, Healthcare Distribution Alliance (HDA), Heather Bresch, John Gray, Michael Nyenhuis, mylan
Pharmacy, Supplier News

STAMFORD, Conn. – Americares, in partnership with the Healthcare Distribution Alliance (HDA), has presented its annual Power of Partnership Award to Mylan, in recognition of its outstanding commitment to increasing access to health care around the world. Mylan is among Americares most dedicated and longstanding supporters, providing pharmaceutical products for the organization’s health programs for people affected
April 3, 2018 by Bill Schiffner
Heather Bresch, HIV Treatment, mylan, Symfi Triple Combo
Pharmacy, Supplier News

HERTFORDSHIRE, England and PITTSBURGH — Global pharmaceutical company Mylan N.V. recently announced that it will introduce in the U.S. a third cost-saving HIV combination. The U.S. Food and Drug Administration (FDA) approved Symfi (efavirenz, lamivudine and tenofovir disoproxil fumarate) 600 mg/300 mg/300 mg tablets, a once-daily, single-tablet regimen (STR), indicated as a complete regimen for the treatment
December 12, 2017 by Chain Drug Review
Brendan O’Grady, generic versions of Viagra, generic Viagra, Greenstone, mylan, Pfizer, sildenafil citrate tablets, Teva Pharmaceutical Industries, Viagra generic
Leading Headlines, Pharmacy, Supplier News

NEW YORK — The first generic versions of Viagra, Pfizer Inc.’s blockbuster drug for erectile dysfunction, were launched this week in the United States by Teva Pharmaceutical Industries Ltd. and Greenstone LLC. Israel-based Teva said it’s offering sildenafil citrate tablets in dosages of 25 mg, 50 mg and 100 mg. Greenstone, a Peapack, N.J.-based Pfizer subsidiary,
December 1, 2017 by Chain Drug Review
Arun Chandavarkar, Biocon, Genentech, Heather Bresch, Herceptin, Herceptin biosimilar, mylan, Ogivri, trastuzumab, trastuzumab-dkst
Pharmacy, Supplier News

PITTSBURGH and BENGALURU, India — Mylan N.V. has received Food and Drug Administration approval for Ogivri (trastuzumab-dkst), a biosimilar oncology drug co-developed with Biocon Ltd. Mylan said Friday that Ogivri is a biosimilar of Herceptin (trastuzumab) from Genentech, indicated for the treatment of HER2-overexpressing breast cancer and metastatic stomach cancer (gastric or gastroesophageal junction adenocarcinoma).
August 18, 2017 by Chain Drug Review
epinephrine auto-injectors, EpiPen, EpiPen Jr, Heather Bresch, Medicaid Drug Rebate Program, mylan
Pharmacy, Supplier News
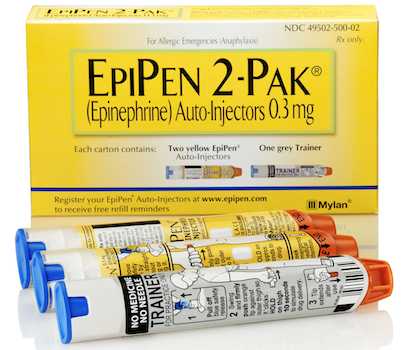
PITTSBURGH — Mylan Inc. and Mylan Specialty L.P. have entered an agreement with the U.S. Department of Justice finalizing the $465 million Medicaid drug rebate settlement last fall. Mylan said late Thursday that the settlement, announced Oct. 7, resolves claims over the classification of the EpiPen and EpiPen Jr epinephrine auto-injectors in the Medicaid Drug
April 25, 2017 by Chain Drug Review
Adrenaclick, Amedra Pharmaceuticals, epinephrine auto-injectors, EpiPen, Jennifer Speares Lehman, Kmart, Kmart Pharmacy, mylan
Leading Headlines, Pharmacy, Retail News

HOFFMAN ESTATES, Ill. — Kmart Pharmacy is tackling the cost issue with epinephrine auto-injectors for anaphylaxis by knocking down the price of a generic version. Kmart said Tuesday that in response to the sharp hikes in price of epinephrine auto-injector pens, it has lowered the cost of the generic version of Adrenaclick to as low












