March 30, 2017 by Chain Drug Review
Cold-EEZE, Heather Bresch, mylan
Business, Pharmacy, Supplier News

PITTSBURGH — Furthering its expansion in the consumer health care arena, Mylan has closed its acquisition of the Cold-EEZE over-the-counter cold remedy line from ProPhase Labs Inc. The transaction, announced in January, gives Mylan all global rights and assets relating to the Cold-EEZE brand, including all U.S. businesses and U.S. and international trademarks. Cold-EEZE’s broad
March 15, 2017 by Chain Drug Review
Aromasin, breast cancer medication, exemestane tablets, generic version of Aromasin, mylan
Pharmacy, Supplier News

PITTSBURGH — Mylan N.V. has launched exemestane tablets 25 mg, a breast cancer medication, in the United States. Mylan said its exemestane product is a generic version of Aromasin tablets from Pfizer Inc. Exemestane is indicated for the treatment of certain types of breast cancer in women after menopause. The medication works by reducing estrogen
February 14, 2017 by Chain Drug Review
AAM, Apotex, Association for Accessible Medicines, biosimilars, Chip Davis, generic drugs, Generic Pharmaceutical Association, GPhA, GPhA Annual Meeting, Heather Bresch, Jeff Watson, Lupin Pharmaceuticals, mylan, Paul McGarty, Peter Goldschmidt, Sandoz
Featured Articles, Leading Headlines, Pharmacy, Supplier News

WASHINGTON — The Generic Pharmaceutical Association (GPhA), which represents manufacturers of generic drugs and biosimilars, has changed its name to the Association for Accessible Medicines (AAM). The association said Tuesday that the new name better reflects its mission: to make medications more accessible to the people who need them. As GPhA, the trade group had steadfastly
February 6, 2017 by Scot Meyer and Chain Drug Review
Adrenaclick, Amedra Pharmaceuticals, CVS Health, CVS Pharmacy, Douglas Boothe, epinephrine auto-injector, EpiPen, Helena Foulkes, Impax Laboratories, mylan
2017, Issue 02-06-2017, Issues, News
WOONSOCKET, R.I. — CVS Health is providing a low-cost alternative Mylan’s EpiPen epinephrine auto-injector for anaphylaxis. CVS has made Impax Laboratories’ epinephrine auto-injector available at all CVS Pharmacy drug stores at a cash price of $109.99 for a two-pack. That compares with a cash price of $649.99 for the branded EpiPen and $339.99 for the
January 12, 2017 by Chain Drug Review
Adrenaclick, Adrenaclick authorized generic, Amedra Pharmaceuticals, Auvi-Q, CVS Health, CVS Pharmacy, Douglas Boothe, epinephrine auto-injector, EpiPen, EpiPen authorized generic, Helena Foulkes, Impax Laboratories, Kaleo, mylan
Leading Headlines, Pharmacy, Retail News
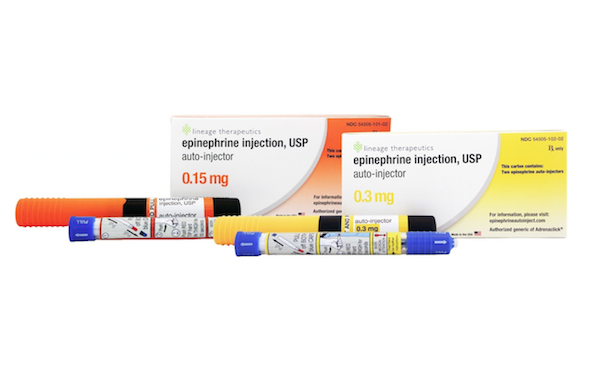
WOONSOCKET, R.I. — In the wake of the public outcry over pricing for Mylan’s EpiPen epinephrine auto-injector for anaphylaxis, CVS Health announced that it’s providing a low-cost alternative. CVS said Thursday that Impax Laboratories’ authorized generic for the Adrenaclick epinephrine auto-injector is now available at all CVS Pharmacy drug stores at a cash price of $109.99
January 10, 2017 by Chain Drug Review
Cold-EEZE, mylan, ProPhase Labs, Rajiv Malik
Business, Leading Headlines, Pharmacy, Supplier News

PITTSBURGH — Mylan N.V. plans to acquire the global rights to the Cold-EEZE over-the-counter cold remedy brand from ProPhase Labs Inc. Under the agreement, Mylan’s U.S. OTC subsidiary will buy substantially all of the assets and other rights relating to the Cold-EEZE brand. Financial terms weren’t disclosed. Cold-EEZE products sold in the U.S. market include
December 30, 2016 by Chain Drug Review
generic version of Concerta, Heather Bresch, medication for attention deficit hyperactivity disorder, methylphenidate hydrochloride extended-release tablets, mylan
Pharmacy, Supplier News

PITTSBURGH — Mylan N.V. has launched methylphenidate hydrochloride extended-release tablets, a medication for attention deficit hyperactivity disorder (ADHD), in the United States. Mylan said its product, a generic version of Concerta tablets from Janssen Pharmaceuticals Inc., comes in dosages of 18 mg, 27 mg, 36 mg and 54 mg. “The launch of generic Concerta tablets
December 20, 2016 by Chain Drug Review
epinephrine auto-injector, generic EpiPen, Heather Bresch, mylan
Pharmacy, Supplier News
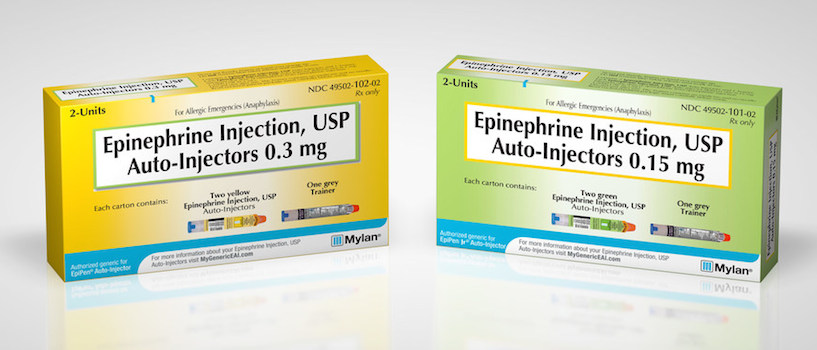
PITTSBURGH — This week, Mylan N.V. began making an authorized generic of its EpiPen epinephrine auto-injector available to pharmacies. Mylan said its generic epinephrine auto-injector, announced in late August, carries a wholesale acquisition cost (WAC) of $300 per two-pack carton, more than 50% lower than the WAC of EpiPen 2-Pak Auto-Injectors. The generic EpiPen, which
October 28, 2016 by Chain Drug Review
generic versions of Benicar and Benicar HCT, medications for high blood pressure, mylan, olmesartan medoxomil tablets, olmesartan medoxomil/hydrochlorothiazide tablets
Pharmacy, Supplier News

PITTSBURGH — Mylan N.V. has announced the U.S. launch of olmesartan medoxomil tablets and olmesartan medoxomil/hydrochlorothiazide tablets, two medications for high blood pressure. Mylan said the products mark the first generic versions of Benicar and Benicar HCT tablets from Daiichi Sankyo. Mylan’s olmesartan medoxomil generic comes in strengths of 5 mg, 20 mg and 40
October 26, 2016 by Chain Drug Review
anaphylaxis, Auvi-Q, epinephrine auto-injector, EpiPen, Eric Edwards, Evan Edwards, generic epinephrine auto-injector, Impax, Kaleo, mylan, Spencer Williamson, video
Leading Headlines, Pharmacy, Supplier News, Videos

RICHMOND, Va. — Pharmaceutical company Kaleo plans to reintroduce the Auvi-Q epinephrine auto-injector to the U.S. market in the first half of 2017. For emergency use in treating life-threatening allergic reactions such as anaphylaxis, Auvi-Q is expected to provide an option to the EpiPen from Mylan Inc., which has been lambasted by government officials, the
September 22, 2016 by Chain Drug Review
EpiPen, Heather Bresch, House Committee on Oversight and Government Reform, mylan, video
Leading Headlines, Videos

Mylan CEO Heather Bresch gave testimony at a hearing Wednesday afternoon by the House Committee on Oversight and Government Reform over the sharp price hike for the EpiPen epinephrine auto-injector since the company acquired the product in 2007. The EpiPen is used for treating anaphylaxis, a potentially life-threatening allergic reaction. Committee chairman Jason Chaffetz (R.,
August 31, 2016 by Chain Drug Review
Adrenaclick, Amedra Pharmaceuticals, anaphylaxis, EpiPen, generic epinephrine auto-injector, Impax Laboratories, mylan
Pharmacy, Supplier News

HAYWARD, Calif. — Impax Laboratories Inc. is spotlighting its generic epinephrine auto-injector amid a public outcry over the cost of emergency medication for anaphylaxis, a potentially life-threatening allergic reaction. In what Impax called an update, the company said it’s providing additional information to patients, doctors and customers about its epinephrine injection auto-injector in 0.15-mg and
August 29, 2016 by Chain Drug Review
anaphylaxis, Chuck Grassley, epinephrine auto-injector, EpiPen, Heather Bresch, mylan
Leading Headlines, Pharmacy, Supplier News
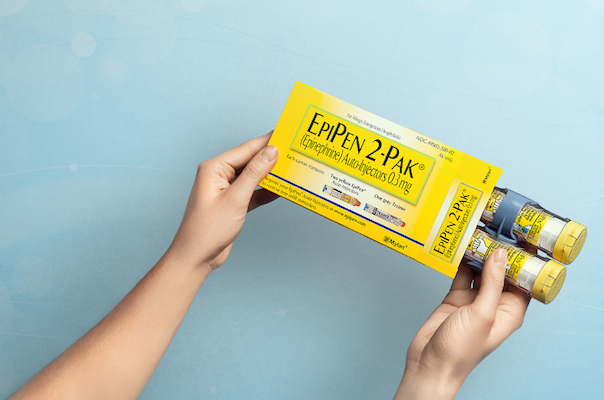
PITTSBURGH — In what chief executive officer Heather Bresch called “an extraordinary commercial response,” Mylan N.V. plans to release a generic version of its EpiPen epinephrine auto-injector for anaphylaxis, answering public demands to lower the cost of the potentially life-saving product. Mylan said Monday that its U.S. subsidiary expects to roll out the generic EpiPen
August 24, 2016 by Chain Drug Review
Amy Klobuchar, anaphylaxis, Chuck Grassley, Claire McCaskill, epinephrine auto-injector, EpiPen, mylan, Richard Blumenthal, Susan Collins
Pharmacy, Supplier News, Uncategorized
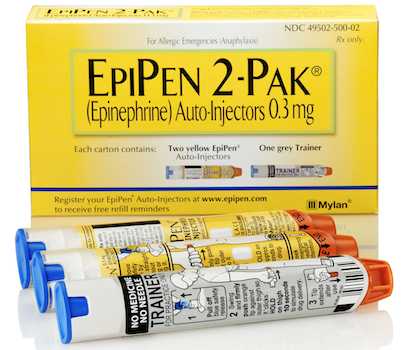
NEW YORK — Senate leaders have voiced concerns about a price surge for Mylan Inc.’s EpiPen, an epinephrine auto-injector for treating anaphylaxis, or potentially life-threatening allergic reactions. Sen. Chuck Grassley (R, Iowa), chairman of the Senate Judiciary Committee, and Sens. Susan Collins (R., Maine) and Claire McCaskill (D., Mo.), chairman and ranking member of the












