April 25, 2018 by Chain Drug Review
app, Novartis, opthalmology
Supplier News

BASEL, Switzerland — Novartis has launched the FocalView app, an ophthalmic digital research platform created with ResearchKit. FocalView aims to allow researchers to track disease progression by collecting real-time, self-reported data directly from consenting patients. By adapting the design of clinical trials to suit the daily routine of patients, the app may reduce barriers to
March 27, 2018 by Chain Drug Review
consumer health care, Emma Walmsley, GlaxoSmithKline, GSK-Novartis Consumer Healthcare, Novartis, Pfizer Consumer Healthcare, Vas Narasimhan
Business, Leading Headlines, Pharmacy, Supplier News
LONDON and BASEL, Switzerland — GlaxoSmithKline plc plans to buy Novartis’ 36.5% stake in their consumer health care joint venture for $13 billion. Under the agreement announced Tuesday, Novartis’ shares in the joint venture will be canceled, and the joint venture will pay the buyout price to Novartis. GSK has entered into a committed facilities agreement to
February 13, 2018 by Chain Drug Review
Copaxone, generic version of Copaxone, glatiramer acetate injection, Glatopa 40 mg/ml, multiple sclerosis medication, Novartis, Richard Francis, Sandoz
Pharmacy, Supplier News

HOLZKIRCHEN, Germany — Following Food and Drug Administration final approval, Sandoz has launched of Glatopa 40 mg/ml (glatiramer acetate injection), a multiple sclerosis medication. The Novartis division said Tuesday that Glatopa 40 mg/ml, manufactured in the United States, was developed under a collaboration pact with Momenta Pharmaceuticals Inc. The product is a fully substitutable generic version of
September 5, 2017 by Chain Drug Review
Joerg Reinhardt, Joseph Jimenez, Novartis, Vasant Narasimhan
Business, Pharmacy, Supplier News

BASEL, Switzerland — Novartis chief executive officer Joseph Jimenez plans to retire from the company next year, to be succeeded by drug development chief Vasant Narasimhan. Plans call for Jimenez to step down on Jan. 31, 2018, and for Narasimhan, who is global head of drug development and chief medical officer at Novartis, to take
June 13, 2017 by Chain Drug Review
Alcon, authorized generic version of Pataday, Novartis, olopatadine hydrochloride ophthalmic solution, Peter Goldschmidt, Sandoz
Pharmacy, Supplier News

PRINCETON, N.J. — Sandoz Inc. has launched olopatadine hydrochloride ophthalmic solution 0.2%, an antihistamine for itchy eyes, in the United States. Sandoz said the product is an authorized generic version of Pataday eye drops from Alcon. Both Sandoz and Alcon are subsidiaries of Novartis. A mast cell stabilizer, olopatadine hydrochloride ophthalmic solution 0.2% is indicated
May 17, 2016 by Chain Drug Review
Bruno Strigini, David Epstein, Joseph Jimenez, Novartis, Novartis Oncology, Novartis Pharmaceuticals, Paul Hudson
Pharmacy, Supplier News

BASEL, Switzerland — Making changes to its Pharmaceuticals Division, Novartis has created two business units that will report to the chief executive officer: Novartis Pharmaceuticals and Novartis Oncology. The new units will form the Innovative Medicines Division. Novartis said Tuesday that the new structure reflects the importance of oncology following the integration of oncology assets
September 3, 2015 by John Schultz and Chain Drug Review
Amgen, filgrastim, Neupogen, Novartis, Richard Francis, Sandoz, Sandoz One Source, Zarxio
Leading Headlines, Pharmacy, Supplier News
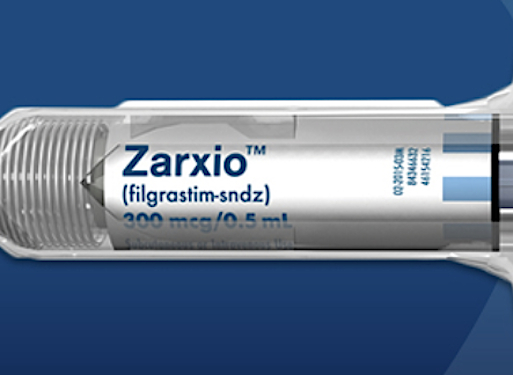
NEW YORK — Sandoz has inaugurated a new era in medicine with the release of Zarxio, the first biosimilar of a biologic drug approved in the United States. The Novartis subsidiary said Thursday that Zarxio — a biosimilar of Amgen Corp.’s oncology drug Neupogen, which boosts white blood cells — would broaden access to an
September 2, 2015 by Chain Drug Review
generic version of the Exelon patch, Novartis, Peter Goldschmidt, rivastigmine patch, rivastigmine transdermal system, Sandoz
Pharmacy, Supplier News

PRINCETON, N.J. — Sandoz has launched a rivastigmine patch, a treatment for dementia, in the U.S. market. The Novartis subsidiary said Wednesday that its rivastigmine transdermal system is an authorized generic version of the Exelon patch, marketed by Novartis Pharmaceuticals Corp. Sandoz is marketing its rivastigmine patch in 13.3-mg strength, the same strength as the
July 28, 2015 by Chain Drug Review
asthma medication, budesonide inhalation suspension, generic version of Pulmicort Respules, Novartis, Peter Goldschmidt, Sandoz
Supplier News
PRINCETON, N.J. — Sandoz has launched budesonide inhalation suspension, an asthma medication, in the U.S. market. The Novartis subsidiary said Tuesday that it will market its budesonide inhalation suspension in 1-mg strength, and it expects 180-day generic drug marketing exclusivity for that dosage. The product is a a generic version of Pulmicort Respules inhalation suspension
July 10, 2015 by Chain Drug Review and Chain Drug Review
Entresto, heart failure, Novartis, sacubitril/valsartan
Pharmacy, Supplier News
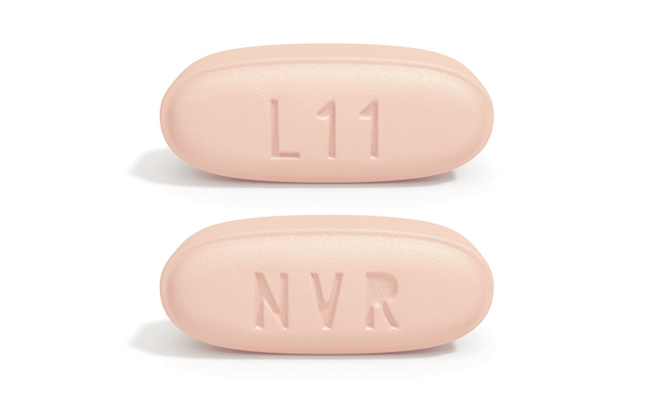
BASEL, Switzerland — Novartis has received Food and Drug Administration approval for Entresto (sacubitril/valsartan) tablets, a medication for heart failure with reduced injection fraction. An angiotensin receptor neprilysin inhibitor (ARNI), Entresto reduces the strain on a failing heart. The twice-a-day tablet acts to enhance the protective neurohormonal systems of the heart while simultaneously suppressing the
March 31, 2015 by Chain Drug Review and Chain Drug Review
amlodipine and valsartan combination tablets, generic version of Exforge, hypertension medication, Novartis, Peter Goldschmidt, Sandoz
Pharmacy, Supplier News
PRINCETON, N.J. — Sandoz Inc. has released amlodipine and valsartan combination tablets, a hypertension medication, in the United States. The company said Tuesday that its product is a generic version of Exforge, made by Novartis. Sandoz will market its combination amlodipine/valsartan tablets in strengths of 5-160 mg, 5-320 mg, 10-160 mg, 10-320 mg, the same








