June 20, 2016 by Chain Drug Review
biologics, biosimilars, Richard Francis, Sandoz, Sandoz Biosimilars Day
Leading Headlines, Pharmacy, Supplier News

HOLZKIRCHEN, Germany — Sandoz aims to launch five biosimilars of major oncology and immunology biologics across key geographies by 2020. The Novartis division said Monday that the new biosimilars will emerge from “an aggressive regulatory submissions strategy” of 11 product filings that began last year and will run through 2017. Plans also call for an
September 3, 2015 by John Schultz and Chain Drug Review
Amgen, filgrastim, Neupogen, Novartis, Richard Francis, Sandoz, Sandoz One Source, Zarxio
Leading Headlines, Pharmacy, Supplier News
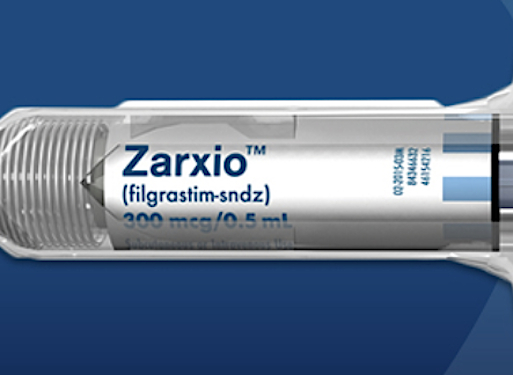
NEW YORK — Sandoz has inaugurated a new era in medicine with the release of Zarxio, the first biosimilar of a biologic drug approved in the United States. The Novartis subsidiary said Thursday that Zarxio — a biosimilar of Amgen Corp.’s oncology drug Neupogen, which boosts white blood cells — would broaden access to an
September 2, 2015 by Chain Drug Review
generic version of the Exelon patch, Novartis, Peter Goldschmidt, rivastigmine patch, rivastigmine transdermal system, Sandoz
Pharmacy, Supplier News

PRINCETON, N.J. — Sandoz has launched a rivastigmine patch, a treatment for dementia, in the U.S. market. The Novartis subsidiary said Wednesday that its rivastigmine transdermal system is an authorized generic version of the Exelon patch, marketed by Novartis Pharmaceuticals Corp. Sandoz is marketing its rivastigmine patch in 13.3-mg strength, the same strength as the
July 28, 2015 by Chain Drug Review
asthma medication, budesonide inhalation suspension, generic version of Pulmicort Respules, Novartis, Peter Goldschmidt, Sandoz
Supplier News
PRINCETON, N.J. — Sandoz has launched budesonide inhalation suspension, an asthma medication, in the U.S. market. The Novartis subsidiary said Tuesday that it will market its budesonide inhalation suspension in 1-mg strength, and it expects 180-day generic drug marketing exclusivity for that dosage. The product is a a generic version of Pulmicort Respules inhalation suspension
June 18, 2015 by Chain Drug Review and Chain Drug Review
Copaxone, generic version of Copaxone, glatiramer acetate injection, Glatopa, Momenta, multiple sclerosis, Sandoz, Teva Pharmaceutical Industries
Pharmacy, Supplier News
HOLZKIRCHEN, Germany — Sandoz has begun shipment of Glatopa, a one-time daily treatment for multiple sclerosis (MS), in the United States. The Novartis subsidiary said Thursday that the product (glatiramer acetate injection, 20 mg/ml) is the first generic version of Copaxone 20 mg, from Teva Pharmaceuticals. Developed in collaboration with Momenta and made in the
April 17, 2015 by Chain Drug Review and Chain Drug Review
Copaxone, Glatopa, multiple sclerosis, Peter Goldschmidt, Sandoz
Supplier News
HOLZKIRCHEN, Germany — Sandoz has received Food and Drug Administration approval for Glatopa, a treatment for multiple sclerosis (MS). The Novartis subsidiary said Thursday that its product is the first generic version of Teva’s Copaxone (glatiramer acetate injection) 20 mg/ml once-daily MS therapy. Glatopa, developed in collaboration with Momenta and made entirely in the United
March 31, 2015 by Chain Drug Review and Chain Drug Review
amlodipine and valsartan combination tablets, generic version of Exforge, hypertension medication, Novartis, Peter Goldschmidt, Sandoz
Pharmacy, Supplier News
PRINCETON, N.J. — Sandoz Inc. has released amlodipine and valsartan combination tablets, a hypertension medication, in the United States. The company said Tuesday that its product is a generic version of Exforge, made by Novartis. Sandoz will market its combination amlodipine/valsartan tablets in strengths of 5-160 mg, 5-320 mg, 10-160 mg, 10-320 mg, the same
March 6, 2015 by John Schultz and Chain Drug Review
biologics, biosimilar, filgrastim, Food and Drug Administration, Margaret Hamburg, Sandoz, Zarxio
News, Pharmacy, Retail News

WASHINGTON — Zarxio (filgrastim), a cancer treatment from Sandoz Inc., has become the first biosimilar product approved in the United States. The Food and Drug Administration announced its approval of Zarxio, a biosimilar to Neupogen, an oncology product from Amgen Inc., on Friday. The move is being hailed as a watershed event that paves the
January 19, 2015 by John Schultz and Chain Drug Review
biosimilar, cancer treatment, Center for Drug Evaluation and Research, FDA, Food and Drug Administration, Generic Pharmaceutical Association, Janet Woodcock, Johnson & Johnson, Merck, Neupogen, Neupogen biosimilar, Ralph Neas, Remicade, rheumatoid arthritis drug, Sandoz, Zarzio
2015, Issue 01-19-2015, News, Pharmacy
WASHINGTON — A Food and Drug Administration panel has recommended that the agency approve what could become the country’s first biosimilar. The panel unanimously urged approval of Sandoz’s filgrastin (Neupogen), a cancer treatment. “This encouraging step forward means that it is very likely now only a question of when, rather than if, filgrastin will be available
January 8, 2015 by Chain Drug Review and Chain Drug Review
biosimilar, filgrastim, John Klimek, National Council for Prescription Drug Programs, NCPDP, Sandoz
CDR Blog, Pharmacy

The National Council for Prescription Drug Programs took part in the meeting that may result in the rollout of the nation’s first biosimilar medication. John Klimek, senior vice president for standards and industry information technology at NCPDP, addressed the Food and Drug Administration’s Center for Drug Evaluation and Research (CDER) and Oncologic Drugs Advisory Committee (ODAC)







