November 12, 2020 by Chain Drug Review
AstraZeneca, C.J. Anderson
Pharmacy, Supplier News
SCOTTSDALE, Ariz. – CCT Research (CCT) announced its partnership with AstraZeneca to advance COVID-19 research. Together with local healthcare providers, CCT will facilitate clinical trials for the AZD1222 COVID-19 VACCINE, an investigational vaccine for the prevention of COVID-19. CCT is recruiting eligible study participants who are 18 years of age or older, not pregnant, in good
September 9, 2020 by Chain Drug Review
AstraZeneca, COVID-19
Leading Headlines, Pharmacy
NEW YORK — AstraZeneca has suspended global trials, including large late-stage trials, of its experimental coronavirus vaccine due to an unexplained illness in a study participant. The vaccine, developed with the University of Oxford, has been described by the World Health Organization as probably the world’s leading candidate and the most advanced in terms of
September 8, 2020 by Chain Drug Review
AstraZeneca, COVID-19, Moderna and Pfizer
Leading Headlines, Supplier News

NEW YORK — According to published reports, nine drug companies pledged Tuesday that they will not submit vaccine candidates for FDA review until their safety and efficacy is shown in large clinical trials. The move is intended to bolster public confidence amid the rush to make a COVID-19 vaccine widely available, and counter fears of
May 22, 2020 by Chain Drug Review
AstraZeneca, COVID-19 vaccine, Oxford University’s vaccine
Leading Headlines, Pharmacy

CAMBRIDGE, U.K. — AstraZeneca is advancing its ongoing response to address the unprecedented challenges of COVID-19, collaborating with a number of countries and multilateral organizations to make the University of Oxford’s vaccine widely accessible around the world in an equitable manner. The company has concluded the first agreements for at least 400 million doses and
May 6, 2020 by Chain Drug Review
AstraZeneca, AstraZeneca’s Farxiga (dapagliflozin), heart failure, Mene Pangalo
Pharmacy, Supplier News

NEW YORK — AstraZeneca’s Farxiga (dapagliflozin) has been approved in the US to reduce the risk of cardiovascular (CV) death and hospitalization for heart failure in adults with heart failure (NYHA class II-IV) with reduced ejection fraction (HFrEF) with and without type 2 diabetes (T2D). The approval by the Food and Drug Administration (FDA) was based
January 1, 2020 by Chain Drug Review
AstraZeneca, Dave Fredrickson, Food and Drug Administration, Lynparza, Pancreatic cancer
Pharmacy, Supplier News

SILVER SPRING, Md. — Pancreatic cancer patients will now have a new treatment option. The Food and Drug Administration has approved the use of AstraZeneca’s Lynparza for patients with tumors that didn’t grow after 16 weeks of chemotherapy. The drug is currently used to treat ovarian and breast cancer. Pancreatic cancer is the third largest cause
January 11, 2018 by Bill Schiffner
AstraZeneca, Bydureon BCise, Rod Wooten, type 2 diabetes, Virginia Valentine
Pharmacy, Supplier News

WILMINGTON, Del. — AstraZeneca announced that Bydureon BCise (exenatide extended-release injectable suspension 2 mg) is now available in pharmacies across the United States. Bydureon BCise was recently approved by the U.S. Food and Drug Administration for adults with type-2 diabetes whose blood sugar remains uncontrolled on one or more oral antidiabetic medicines in addition to
November 16, 2017 by Chain Drug Review
AstraZeneca, benralizumab, Fasenra, MedImmune, Pascal Soriot
Pharmacy, Supplier News
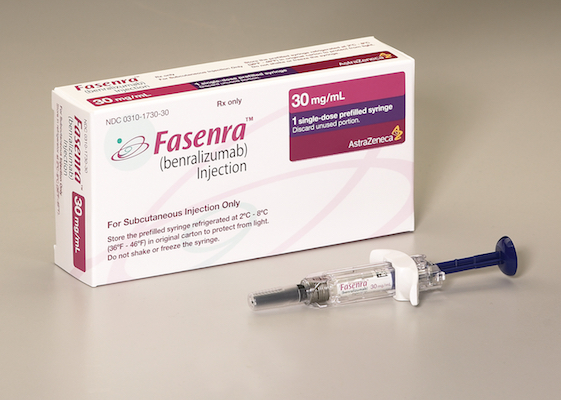
WILMINGTON, Del. — AstraZeneca plans to release Fasenra, its newly approved biologic drug for severe asthma, to U.S. pharmacies in the coming weeks. Fasenra (benralizumab), approved this week by the Food and Drug Administration, is indicated for the add-on maintenance treatment of patients ages 12 and older with severe asthma and an eosinophilic phenotype. AstraZeneca said
October 24, 2017 by Chain Drug Review
AstraZeneca, AstraZeneca US, Bydureon, Bydureon BCise, exenatide, Ruud Dobber
Pharmacy, Supplier News
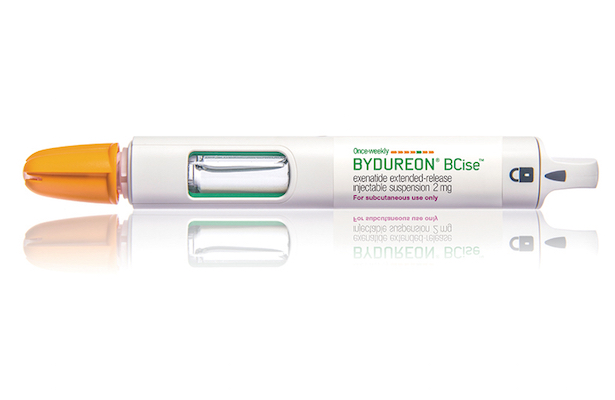
WILMINGTON, Del. — Early next year, AstraZeneca plans to release Bydureon BCise, a new formulation of Bydureon (exenatide extended-release) injectable suspension in an improved once-weekly, single-dose autoinjector for adults with type 2 diabetes. The company said this week that it has received Food and Drug Administration approval for Bydureon BCise, indicated to improve glycemic
October 18, 2017 by Chain Drug Review
AstraZeneca, Aurobindo Pharma, generic version of Nexium 24HR, Nexium 24HR, Pfizer Consumer Healthcare
Pharmacy, Supplier News

HYDERABAD, India — Aurobindo Pharma Ltd. has received Food and Drug Administration approval to manufacture over-the-counter 20-mg esomeprazole magnesium delayed-release capsules, an antacid. Aurobindo said its esomeprazole OTC product, being shipped immediately, is a generic version of Nexium 24HR capsules from Pfizer Consumer Healthcare. A proton pump inhibitor (PPI), Nexium 24HR capsules are indicated for
November 1, 2016 by Chain Drug Review
AstraZeneca, Camber Pharmaceuticals, cholesterol-lowering medication, generic version of Crestor, rosuvastatin tablets
Pharmacy, Supplier News

PISCATAWAY, N.J. — Camber Pharmaceuticals has released rosuvastatin tablets, a cholesterol-lowering medication. Camber said Monday that its product, a generic version of Crestor tablets from AstraZeneca Pharmaceuticals, comes in strengths of 5 mg, 10 mg, 20 mg and 40 mg. The 5-, 10- and 20-mg dosages are available in 90-count bottles, while the 40-mg dosages is
May 3, 2016 by Chain Drug Review
Allergan, AstraZeneca, generic version of Crestor, Robert Stewart, rosuvastatin calcium
Pharmacy, Supplier News

DUBLIN, Ireland — Allergan plc has received final approval from the Food and Drug Administration for rosuvastatin calcium tablets, a cholesterol-lowering medication. The company said its rosuvastatin calcium product comes in dosages of 5 mg, 10 mg, 20 mg and 40 mg and is a generic version of Crestor tablets from AstraZeneca. Allergan noted that
December 17, 2015 by Chain Drug Review
AstraZeneca, chronic obstructive pulmonary disease, COPD, Daliresp, Takeda
Pharmacy, Supplier News

LONDON and OSAKA, Japan — AstraZeneca plans to acquire the core respiratory business of Takeda Pharmaceutical Co. Ltd. for $575 million. Under the agreement, approximately 200 Takeda staff will transfer to AstraZeneca after the closing of the transaction, expected in the first quarter of 2016. AstraZeneca said the deal will include the expansion of rights
September 30, 2015 by Chain Drug Review
AstraZeneca, Brilinta, Brilinta 60 mg, ticagrelor, Tonous Silfani
Pharmacy, Supplier News

WILMINGTON, Del. — AstraZeneca has released Brilinta (ticagrelor) 60-mg tablets, a smaller dose of the anticoagulant medication, to U.S. pharmacies. The pharmaceutical company said Tuesday that the Food and Drug Administration approved the new 60-mg strength for Brilinta for use in patients with a history of heart attack beyond the first year. The dosing of














