January 1, 2020 by Chain Drug Review
AstraZeneca, Dave Fredrickson, Food and Drug Administration, Lynparza, Pancreatic cancer
Pharmacy, Supplier News

SILVER SPRING, Md. — Pancreatic cancer patients will now have a new treatment option. The Food and Drug Administration has approved the use of AstraZeneca’s Lynparza for patients with tumors that didn’t grow after 16 weeks of chemotherapy. The drug is currently used to treat ovarian and breast cancer. Pancreatic cancer is the third largest cause
August 15, 2019 by Shannon Cox and Stephen Cummings
2018 Farm Bill, cannabis, Epidiolex, Food and Drug Administration
August 12, 2019, Opinion

There is a growing public focus on the potential for legitimate pharmacological uses of cannabis. Much of this focus has been directed toward medical marijuana — the use of marijuana in plant form or its basic extracts. Although some states have legalized its use, the Food and Drug Administration has not approved marijuana in plant
May 31, 2019 by Chain Drug Review
CBD (cannabidiol), Food and Drug Administration, Food Marketing Institute (FMI), Leslie Sarasin
Leading Headlines, Retail News

WASHINGTON – Food Marketing Institute (FMI) on Friday participated in the Food and Drug Administration’s (FDA) public hearing regarding products containing cannabis or cannabis-derived compounds, including hemp and cannabidiol (CBD), as the agency considers whether and how to create a legal pathway for such products. FMI both conveyed the seriousness of the regulatory ambiguity facing its
February 11, 2019 by Chain Drug Review
Commissioner, Council for Responsible Nutrition (CRN), Food and Drug Administration, president and chief executive officer at CRN, Scott Gottlieb, Steve Mister
Leading Headlines

WASHINGTON — In response to a statement published by Scott Gottlieb, Commissioner, Food and Drug Administration on February 11, the Council for Responsible Nutrition (CRN), the leading trade association for the dietary supplement and functional food industry, issued the following statement: Statement by Steve Mister, president and chief executive officer at CRN: “CRN appreciates the
December 21, 2018 by Chain Drug Review
cannabidiol (CBD), FDA Commissioner Scott Gottlieb, Food and Drug Administration
Leading Headlines

SILVER SPRING, Md. — Yesterday, FDA Commissioner Scott Gottlieb issued the following statement on the signing of the Agriculture Improvement Act and the agency’s regulation of products containing cannabis and cannabis-derived compound: “Today, the Agriculture Improvement Act of 2018 was signed into law. Among other things, this new law changes certain federal authorities relating to
November 9, 2018 by Chain Drug Review
e-cigarettes, Food and Drug Administration, Scott Gottlieb
Leading Headlines, Retail News

SILVER SPRING, Md. — Seeking to curb vaping by minors, the Food and Drug Administration is banning retail sales of most flavored e-cigarettes. FDA Commissioner Scott Gottlieb early this month was on the verge of announcing the prohibition (which will exempt menthol and mint flavors), as well as imposing age-verification requirements for online e-cigarette sales.
April 17, 2018 by Chain Drug Review
Amazon, FDA, Food and Drug Administration
Issue 04-09-2018, News
SEATTLE — Amazon.com has hired Taha Kass-Hout, the Food and Drug Administration’s first chief health informatics officer, according to published reports. Kass-Hout will serve in a business development role with the company’s Amazon Grand Challenge team, the reports say, and will concentrate on developing projects for the health care market. His exact mission, like the
February 27, 2018 by Chain Drug Review
2017-2018 flu vaccine, Centers for Medicare & Medicaid Services, effectiveness of the flu vaccine, Food and Drug Administration, influenza A H3N2, Scott Gottlieb, World Health Organization
Leading Headlines, Pharmacy, Retail News
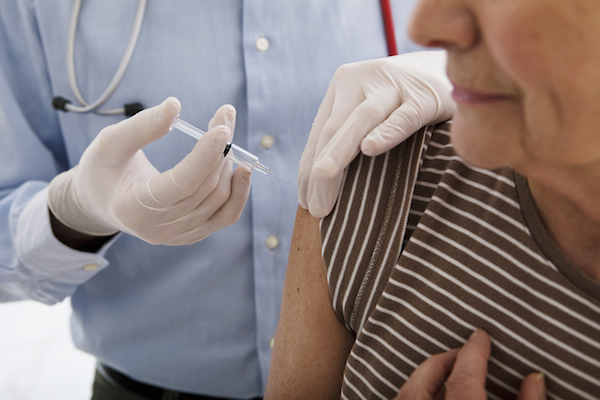
SILVER SPRING, Md. — The Food and Drug Administration has begun efforts to improve flu vaccine effectiveness, according to FDA commissioner Scott Gottlieb. In a statement released yesterday, Gottlieb cited less protection against the influenza A H3N2 virus as a chief reason for the low efficacy of the 2017-2018 flu vaccine. The Centers for Disease
February 12, 2018 by Greg Jacobson and Chain Drug Review
Alex Azar, Eli Lilly, Food and Drug Administration, HHS secretary, prescription drug prices, President Donald Trump, Secretary of Health and Human Services, State of the Union, Trump's State of the Union address
2018, Issue 02-12-2018, Issues, Leading Headlines, News, Pharmacy, Retail News

WASHINGTON — In his first State of the Union speech, President Donald Trump renewed his pledge to reduce prescription drug prices, days after a former big pharma executive was sworn in as Secretary of Health and Human Services. Trump had stunned the pharmaceutical industry during his 2016 campaign by declaring that drug manufacturers were “getting
September 25, 2017 by Bill Schiffner
Food and Drug Administration, illegal online pharmacies, International Internet Week of Action, Operation Pangea X, rogue online pharmacies, Scott Gottlieb
Leading Headlines, Pharmacy, Retail News

SILVER SPRINGS, Md. — The Food and Drug Administration has clamped down on hundreds of websites engaged in illegal sales of prescription drugs. The FDA said Monday that, in tandem with international regulatory and law enforcement agencies, actions were taken against more than 500 websites selling potentially dangerous, unapproved versions of prescription medicines — including
June 5, 2017 by Greg Jacobson and Chain Drug Review
Centers for Disease Control and Prevention, cuts in health care, Department of Health and Human Services, fiscal 2018 U.S. budget, Food and Drug Administration, Mick Mulvaney, National Institutes of Health
2017, Issue 06-05-2017, Issues, News
WASHINGTON — The fiscal 2018 U.S. budget issued by the Trump administration calls for steep cuts in health care and scientific research while heavily boosting defense spending and offering substantial tax cuts. Dubbed “A New Foundation for American Greatness,” the spending plan was touted as a “taxpayer first” budget by Mick Mulvaney, director of the
May 11, 2017 by Chain Drug Review
Biotechnology Innovation Organization, commissioner of the Food and Drug Administration, FDA commissioner, Food and Drug Administration, Jim Greenwood, Lamar Alexander, Robert Califf, Scott Gottlieb, Stephen Ostroff, Tom Price, U.S. Department of Health and Human Services
Featured Articles, Leading Headlines, Pharmacy, Retail News

WASHINGTON — Dr. Scott Gottlieb has been sworn in as the new commissioner of the Food and Drug Administration. Gottlieb, nominated as FDA commissioner by President Donald Trump in March, took the helm as the FDA’s 23rd commissioner on Thursday after being confirmed by a vote of 57-42 in the Senate late Tuesday. He takes
October 3, 2016 by Chain Drug Review
Baby Orajel, CVS, FDA, Food and Drug Administration, homeopathic teething tablets and gels, Hyland’s, Janet Woodcock
Leading Headlines, Supplier News

SILVER SPRING, Md. — The Food and Drug Administration has issued a warning that homeopathic teething tablets and gels may present a health risk to infants and children. The FDA on Friday urged consumers to stop using the products and dispose of any that they have. The agency said the homeopathic teething tablets and gels
September 6, 2016 by Chain Drug Review
antibacterial hand and body wash products, antiseptic soaps, FDA's Center for Drug Evaluation and Research, Food and Drug Administration, Janet Woodcock, Theresa Michele, triclocarban, triclosan
Leading Headlines, Supplier News

WASHINGTON — Over-the-counter consumer antiseptic soaps with certain active ingredients can no longer be marketed, according to a final rule issued by the Food and Drug Administration. The FDA said the final rule, published Tuesday, applies to consumer antibacterial hand and body wash products containing one or more of 19 active ingredients, including triclosan and













