September 8, 2022 by Chain Drug Review
FDA, HDA
Pharmacy

ARLINGTON, Va. — The Healthcare Distribution Alliance (HDA) released the following statement on the U.S. Food and Drug Administration’s (FDA) proposed national licensure standards for distributors and third-party logistics providers (3PLs). HDA recently submitted public comments to FDA on the agency’s proposed standards, which are required to be established under the 2013 Drug Supply Chain Security Act (DSCSA).
August 16, 2022 by Chain Drug Review
FDA, Hearing Aids, OTC Hearing Aid Act
Leading Headlines, Supplier News

WASHINGTON — On Tuesday, the U.S. Food and Drug Administration issued a final rule to improve access to hearing aids which may in turn lower costs for millions of Americans. This action establishes a new category of over-the-counter (O-T-C) hearing aids, enabling consumers with perceived mild to moderate hearing impairment to purchase hearing aids directly
July 6, 2022 by Chain Drug Review
FDA, Paxlovid (nirmatrelvir and ritonavir)
Leading Headlines, Pharmacy

WASHINGTON — The U.S. Food and Drug Administration on Wednesday revised the Emergency Use Authorization (EUA) for Paxlovid (nirmatrelvir and ritonavir), to authorize state-licensed pharmacists to prescribe Paxlovid to eligible patients, with certain limitations to ensure appropriate patient assessment and prescribing of Paxlovid. “The FDA recognizes the important role pharmacists have played and continue to play
April 26, 2022 by Eva Temkin and Christina Markus
Christina Markus and Eva Temkin are attorneys in King & Spalding’s Washington office, FDA
April 25, 2022, Opinion

More than 30 years ago, U.S. law mandated state licensure of prescription drug wholesale distributors, resulting in a patchwork of varying state requirements and practical burdens for multi-state businesses. In 2013, Congress passed the Drug Supply Chain Security Act (DSCSA), which was intended to strengthen the integrity of the pharmaceutical distribution supply chain and to
March 29, 2022 by Chain Drug Review
COVID booster shot, FDA
Leading Headlines, Pharmacy

SILVER SPRING, Md. — The U.S. Food and Drug Administration authorized on Tuesday a second booster dose of either the Pfizer-BioNTech or the Moderna COVID-19 vaccines for older people and certain immunocompromised individuals. The FDA previously authorized a single booster dose for certain immunocompromised individuals following completion of a three-dose primary vaccination series. This action
February 23, 2022 by Chain Drug Review
Alliance for Pharmacy Compounding, FDA
Pharmacy

ALEXANDRIA, Va. — In a significant legal victory for pharmacy compounders, the U.S. Food & Drug Administration has conceded that it must conduct formal notice-and-comment rulemaking to implement its memorandum of understanding with states regarding interstate shipments of compounded medications. The agency concession is related to a lawsuit, Wellness Pharmacy v. Azar, brought in federal court against
February 15, 2022 by Chain Drug Review
Dr. Robert Califf, FDA
Leading Headlines, Pharmacy

WASHINGTON — The Senate on Tuesday narrowly confirmed Dr. Robert Califf as commissioner of the Food and Drug Administration, a key federal agency that has been without a permanent chief for more than a yearlong stretch of the coronavirus pandemic. The vote was 50-46, with six Republicans crossing the aisle to support him while five Democrats opposed
January 19, 2022 by Chain Drug Review
CHPA, FDA
Leading Headlines, Supplier News

WASHINGTON — With a growing number of Americans taking dietary supplements for self-care and preventive health management, the Consumer Healthcare Products Association (CHPA) Educational Foundation launched an interactive Supplement Facts label on its consumer-facing website KnowYourOTCs.org to highlight for shoppers what information they can find on a Supplement Facts label. All dietary supplements are required by
January 3, 2022 by Chain Drug Review
FDA
Leading Headlines, Pharmacy
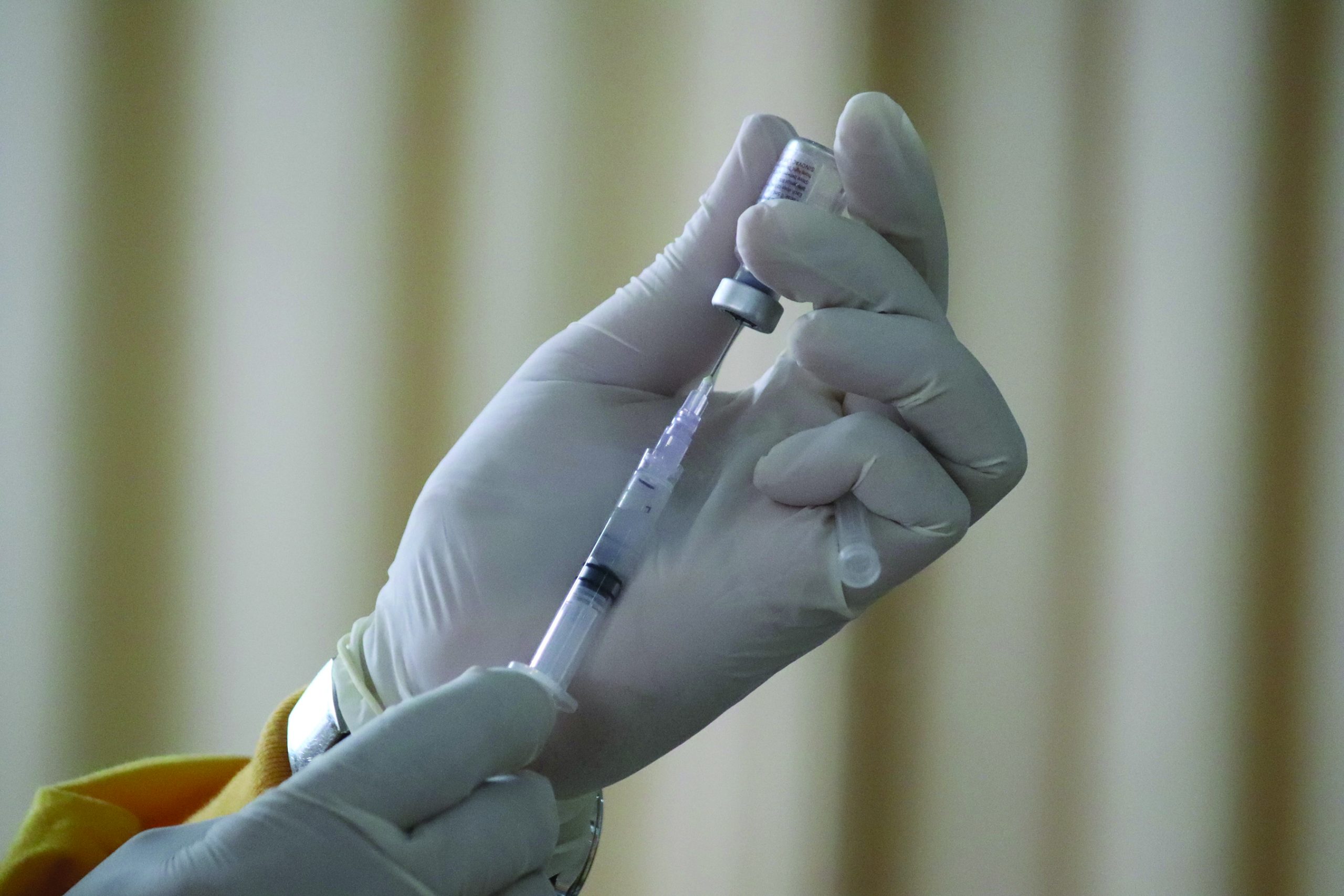
WASHINGTON — The U.S. is expanding COVID-19 boosters as it battles the omicron surge, with the Food and Drug Administration allowing extra Pfizer shots for children as young as 12. Boosters already are recommended for everyone 16 and older, and federal regulators on Monday decided they’re also warranted for 12- to 15-year-olds once enough time
December 22, 2021 by Chain Drug Review
FDA, Pfizer’s Paxlovid (nirmatrelvir tablets and ritonavir tablets
Leading Headlines, Pharmacy

WASHINGTON— U.S. health regulators on Wednesday authorized the first pill against COVID-19, a Pfizer drug that Americans will be able to take at home to head off the worst effects of the virus. The U.S. Food and Drug Administration issued an emergency use authorization (EUA) for Pfizer’s Paxlovid (nirmatrelvir tablets and ritonavir tablets, co-packaged for
November 19, 2021 by Chain Drug Review
FDA
Leading Headlines, Pharmacy

WASHINGTON — The Food and Drug Administration has moved to expand its emergency authorization of Pfizer’s and Moderna’s COVID-19 vaccine booster shots to all adults, the companies announced Friday, as health officials are clamoring to head off a potential new surge of cases this winter. The Centers for Disease Control and Prevention must also weigh in with its recommendations before
October 29, 2021 by Chain Drug Review
COVID-19 vaccine, FDA
Leading Headlines, Pharmacy

WASHINGTON — The Food and Drug Administration on Friday paved the way for children ages 5 to 11 to get Pfizer’s COVID-19 vaccine. The FDA cleared kid-size doses — just a third of the amount given to teens and adults — for emergency use, and up to 28 million more American children could be eligible
October 26, 2021 by Chain Drug Review
FDA, fizer/BioNTech COVID-19 vaccine for children
Leading Headlines, Pharmacy

WASHINGTON – An expert panel on Tuesday voted overwhelmingly to recommend the U.S. Food and Drug Administration authorize the Pfizer and BioNTech SE COVID-19 vaccine for children ages 5 to 11, saying the benefits of inoculation outweigh the risks. An authorization for that age group would be would be an important regulatory step toward reaching
October 20, 2021 by Chain Drug Review
COVID booster shot, FDA
Leading Headlines, Pharmacy

WASHINGTON — The FDA has authorized booster doses of both COVID-19 vaccines made by Moderna and Johnson & Johnson Wednesday and also said any of the three authorized vaccines could be used as a booster in a “mix and match” approach. The FDA gave emergency use authorization for a half dose of Moderna’s vaccine as








