August 31, 2015 by Chain Drug Review
Academy of Managed Care Pharmacy, AMCP, Bertrand Liang, biologic drugs, biologic products, Biologics Prescribers Collaborative, biosimilar product names, biosimilars, Biosimilars Forum, Center for Biologics Evaluation and Research, Center for Drug Evaluation and Research, Edith Rosato, Food and Drug Administration, GPhA, Janet Woodcock, Karen Midthun, The Biosimilars Council
Featured Articles, Leading Headlines, Pharmacy, Supplier News

WASHINGTON — Early feedback on the Food and Drug Administration’s proposal on naming conventions for biosimilars shows a mixed response. Late late week, the FDA published draft guidance recommending that reference biologic products and biosimilars have nonproprietary names that share a core drug substance name and an FDA-designated suffix unique for each product. For interchangeable
August 19, 2015 by Chain Drug Review
Addyi, CDER, Center for Drug Evaluation and Research, Cindy Whitehead, female sexual dysfunction, Food and Drug Administration, HSDD, hypoactive sexual desire disorder, Janet Woodcock, Sprout Pharmaceuticals
Pharmacy, Supplier News
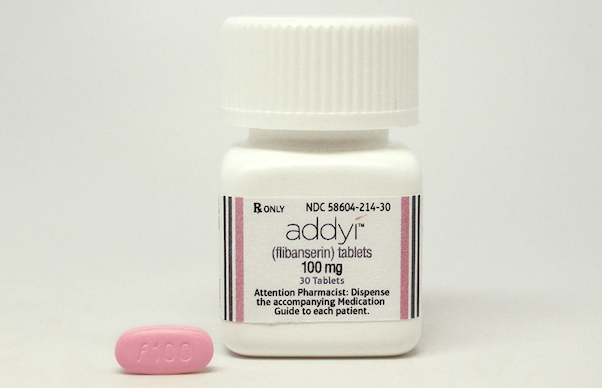
RALEIGH, N.C. — Sprout Pharmaceuticals Inc. has gained Food and Drug Administration clearance to market Addyi, which the company calls the first FDA-approved medication for hypoactive sexual desire disorder (HSDD) in women. Sprout said late Tuesday that Addyi (flibanserin, 100 mg) is slated to be released by Oct. 17. “It has been a remarkable journey to
July 7, 2015 by Chain Drug Review and Chain Drug Review
Center for Drug Evaluation and Research, cystic fibrosis, Cystic Fibrosis Foundation, Food and Drug Administration, Jeffrey Leiden, John Jenkins, lumacaftor/ivacaftor, Office of New Drugs, Orkambi, Robert Beall, Vertex Pharmaceuticals
Pharmacy, Supplier News
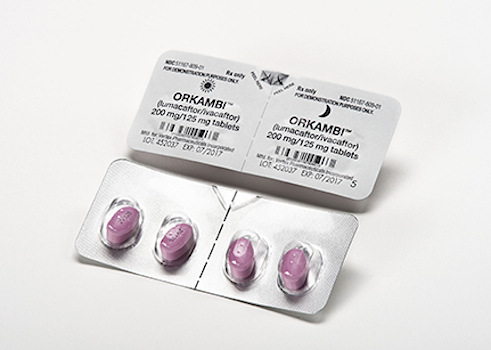
BOSTON — The approval of Vertex Pharmaceuticals Inc.’s Orkambi (lumacaftor/ivacaftor) is being hailed as a big stride forward in the treatment of cystic fibrosis. The Food and Drug Administration announced its approval of the drug just before the holiday weekend. Vertex noted that the medication is the first to treat the underlying cause of cystic fibrosis in
January 19, 2015 by John Schultz and Chain Drug Review
biosimilar, cancer treatment, Center for Drug Evaluation and Research, FDA, Food and Drug Administration, Generic Pharmaceutical Association, Janet Woodcock, Johnson & Johnson, Merck, Neupogen, Neupogen biosimilar, Ralph Neas, Remicade, rheumatoid arthritis drug, Sandoz, Zarzio
2015, Issue 01-19-2015, News, Pharmacy
WASHINGTON — A Food and Drug Administration panel has recommended that the agency approve what could become the country’s first biosimilar. The panel unanimously urged approval of Sandoz’s filgrastin (Neupogen), a cancer treatment. “This encouraging step forward means that it is very likely now only a question of when, rather than if, filgrastin will be available





